Major uses of distillation
in the food industry are for concentrating essential oils, flavours and
alcoholic beverages, and in the deodorization of fats and oils.
The
equilibrium relationships in distillation are governed by the relative
vapour pressures of the mixture components, that is by their volatility
relative to one another. The equilibrium curves for two-component vapour-liquid
mixtures can conveniently be presented in two forms, as boiling temperature/concentration
curves, or as vapour/liquid concentration distribution curves. Both forms
are related as they contain the same data and the concentration distribution
curves, which are much the same as the equilibrium curves used in extraction,
can readily be obtained from the boiling temperature/concentration curves.
A
boiling temperature/concentration diagram is shown in Fig. 9.10.
Notice that there are two curves on the diagram, one giving the liquid
concentrations and the other the vapour concentrations.
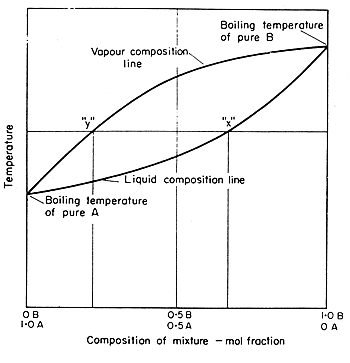
Figure 9.10 Boiling temperature/concentration diagram
If a horizontal (constant
temperature) line is drawn across the diagram within the limit temperatures
of the two curves, it will cut both curves. This horizontal line corresponds
to particular boiling temperature, the point at which it cuts the lower
line gives the concentration of the liquid boiling at this temperature,
the point at which it cuts the upper line gives the concentration of the
vapour condensing at this same temperature. Thus the two points give the
two concentrations which are in equilibrium. They give in fact two corresponding
values on the concentration distribution curves, the point on the liquid
line corresponding to an x point (that is to the concentration
in the heavier phase) and the point on the vapour line to a y point
(concentration in the lighter phase). The diagram shows that the y
value is richer in the more volatile component of the mixture than x,
and this is the basis for separation by distillation.
It
is found that some mixtures have boiling-point diagrams that are a
different shape from
that
shown in Fig. 9.10. For these mixtures, at another particular temperature
and away from the pure components at the extremes of composition, the
vapour and liquid composition lines come together. This means that,
at
this temperature, the liquid boils to give a vapour of the same composition
as itself. Such mixtures constitute azeotropes
and their formation limits the concentration attainable in a distillation
column. The ethanol-water mixture, which is of great importance in the
alcoholic beverage industry, has a minimum boiling-point azeotrope at
composition 89.5 mole% (95.6% w/w) ethanol and 10.5 mole% (4.4% w/w)
water,
which boils at 78.15°C. In a distillation column, separating dilute
ethyl alcohol and water, the limit concentrations of the streams are
100% water
on the one hand in the "liquid" stream, and 95.6% ethyl alcohol,
4.4% water by weight in the "vapour" stream, however many
distillation stages are used.
A
multi-stage distillation column works by providing successive stages
in which liquids boil and
the vapours from the stage above condense and in which equilibrium between
the two streams, liquid and vapour, is attained. Mass balances can
be
written for the whole column, and for parts of it, in the same way as
with other contact equilibrium processes.
 EXAMPLE 9.13. Distillation of alcohol/water mixture EXAMPLE 9.13. Distillation of alcohol/water mixture
In a single-stage, continuous distillation column used for enriching alcohol/water
mixtures, the feed contains 12% of alcohol, and 25% of the feed passes
out with the top product (the "vapour" stream) from the still.
Given that, at a boiling temperature of 95.5°C, 1.9 mole% of alcohol
in the liquid is in equilibrium with 17 mole% of alcohol in the vapour,
estimate the concentration of alcohol in the product from the still.
From
the equilibrium data given and since the mole fraction of alcohol is
small we may assume
a linear equilibrium relationship. The equilibrium curve passes through
(0,0) and (1.9, 17) so that over this range we can say y = x(17/1.9)
or x = y(1.9/17).
From the operating
conditions given, as the feed is equal to liquid + vapour phases,(L
+ V) we can write:
F = L + V and also
V = F/4 and so
F = 4V and L = 3V
Therefore, for the alcohol, if xf is the concentration
in the feed, we can write a mass balance across the distillation column:
4Vxf =3Vx + Vy
The concentration
of alcohol in the feed is 12% which has to be expressed as a mole fraction
to be in the same units as the equilibrium data. The molecular weight
of alcohol is 46 (C2H5OH), and of water 18.
xf =
(12/46)/(88/18 + 12/46)
= 0.05
Operating equation:
4 x 0.05
= 3x + y
Equilibrium condition
x =
(1.9/17)y,
y(3 x 1.9/17) + y = 0.2
And so y =
0.15 mole%
Letting the weight
fraction of alcohol in the vapour stream be w we have:
0.15
= (w/46)/(w/46
+ (1 - w)/18)
so w = 0.31 = 31%
The concentration of alcohol in product from still = 31%
Continuous fractional
distillation columns can be analysed in rather similar ways to continuous
extraction systems, They generally have a reboiler at one end of a column
and a condenser at the other (head). A feed stream normally enters somewhere
away from the end points of the column and there is often provision of
reflux which is a distillate return flow from the condenser section at
the head of the column. Full analysis of such columns can be found in
standard chemical engineering texts.
Steam Distillation
In some circumstances
in the food industry, distillation would appear to be a good separation
method but it cannot be employed directly as the distilling temperatures
would lead to breakdown of the materials. In cases in which volatile materials
have to be removed from relatively non-volatile materials, steam distillation
may sometimes be used to effect the separation at safe temperatures.
A liquid boils when
the total vapour pressure of the liquid is equal to the external pressure
on the system. Therefore, boiling temperatures can be reduced by reducing
the pressure on the system; for example by boiling under a vacuum, or
by adding an inert vapour which by contributing to the vapour pressure,
allows the liquid to boil at a lower temperature. Such an addition must
be easily removed from the distillate, if it is unwanted in the product,
and it must not react with any of the components that are required as
products. The vapour that is added is generally steam and the distillation
is then spoken of as steam distillation.
If the vapour pressure
of the introduced steam is ps and the total pressure is P,
then the mixture will boil when the vapour pressure of the volatile component
reaches a pressure of
(P - ps), compared with the necessary pressure
of P if there were
no steam present. The distribution of steam and the volatile component
being distilled, in the vapour, can be calculated. The ratio of the
number
of molecules of the steam to those of the volatile component, will be
equal to the ratio of their partial pressures
pA/ps
= (P - ps)/ps = (wA/MA)/(ws/Ms)
(9.23)
and so the weight
ratios can be written:
wA/wS
= (P - ps)/ps x (MA/Ms)
(9.22)
where pA is
the partial pressure of the volatile component, ps is the partial
pressure of the steam, P is the total pressure on the system, wA
is the weight of component A in the vapour, ws is the weight of steam in the vapour, MA is the molecular
weight of the volatile component and Ms is the molecular weight
of steam.
Very often the molecular
weight of the volatile component that is being distilled is much greater
than that of the steam, so that the vapour may contain quite large proportions
of the volatile component. Steam distillation is used in the food industry
in the preparation of some volatile oils and in the removal of some taints
and flavours, for example from edible fats and oils.
Vacuum Distillation
Reduction
of the total pressure in the distillation column provides another
means of distilling
at lower temperatures. When the vapour pressure of the volatile substance
reaches the system pressure, distillation occurs. With modern efficient
vacuum-producing equipment, vacuum distillation is tending to supplant
steam distillation. In some instances, the two methods are combined
in
vacuum steam distillation.
Batch Distillation
Batch distillation
is the term applied to equipment into which the raw liquid mixture is
admitted and then boiled for a time. The vapours are condensed. At the
end of the distillation time, the liquid remaining in the still is withdrawn
as the residue. In some cases the distillation is continued until the
boiling point reaches some predetermined level, thus separating a volatile
component from a less volatile residue. In other cases, two or more fractions
can be withdrawn at different times and these will be of decreasing volatility.
During batch distillation, the concentrations change both in the liquid
and in the vapour.
Let L be the
mols of material in the still and x be the concentration of the
volatile component. Suppose an amount dL is vaporized, containing a fraction
y of the volatile component.
Then writing a material
balance on component A, the volatile component:
ydL
= d(Lx) = Ldx + xdL
dL/L = dx/(y - x)
and this is to be
integrated from L0 moles of material of concentration x0
up to L moles at concentration x.
To evaluate this
integral, the relationship between x and y, that is the
equilibrium conditions, must be known.
If the equilibrium
relationship is a straight line, y = mx + c, then the integral
can be evaluated:
LogeL/Lo
= 1 Loge
(m - 1)x + c
(m - 1)
(m - 1)xo + c
(9.21)
or
L/Lo = [(y - x)/(yo - xo)]1/(m-1)
In general, the
equilibrium relationship is not a straight line, and the integration has
to be carried out graphically. A graph is plotted of x against 1/(y
- x), and the area under the curve between values of x0 and
x is measured.
Distillation Equipment
The conventional
distillation equipment for the continuous fractionation of liquids consists
of three main items: a boiler in which the necessary heat to vaporize
the liquid is supplied, a column in which the actual contact stages for
the distillation separation are provided, and a condenser for condensation
of the final top product. A typical column is illustrated in Fig. 9.11.
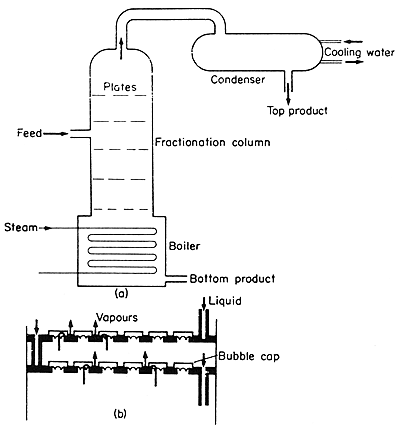
Figure 9.11 Distillation column (a) assembly, (b) bubble-cap trays
The condenser and
the boiler are straightforward. The fractionation column is more complicated
as it has to provide a series of contact stages for contacting the liquid
and the vapour. The conventional arrangement is in the form of "bubble-cap"
trays, which are shown in Fig. 9.11(b). The vapours rise through the bubble
caps. The liquid flows across the trays past the bubble caps where it
contacts the vapour and then over a weir and down to the next tray. Each
tray represents a contact stage, or approximates to one as full equilibrium
is not necessarily attained, and a sufficient number of stages must be
provided to reach the desired separation of the components.
In steam distillation,
the steam is bubbled through the liquid and the vapours containing the volatile
component and the steam are passed to the condenser. Heat may be provided
by the condensation of the steam, or independently. In some cases the steam
and the condensed volatile component are immiscible, so that separation
in the condenser is simple.
 Contact-Equilibrium
Processes - SUMMARY, PROBLEMS Contact-Equilibrium
Processes - SUMMARY, PROBLEMS
 Back
to the top Back
to the top |
![]() Steam
Distillation
Steam
Distillation![]() Vacuum Distillation
Vacuum Distillation![]() Batch Distillation
Batch Distillation![]() Distillation Equipment
Distillation Equipment

