CHAPTER
9
CONTACT EQUILIBRIUM PROCESSES - THEORY
(cont'd)
SOLID/LIQUID EQUILIBRIA
Liquids have a capacity
to dissolve solids up to an extent, which is determined by the
solubility of the particular solid material in that liquid.
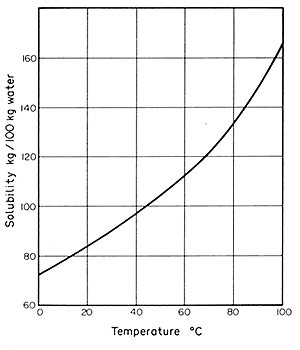
Solubility is a function of temperature and, in most cases, solubility
increases with rising temperature. A solubility curve can be drawn to
show this relationship, based on the equilibrium concentration in solution
measured in convenient units, for example gkg-1, as a function
of temperature. Such a curve is illustrated in Fig. 9.1 for sodium
nitrite in water.