Losses
of moisture may also occur when they are not desired, for example during
curing of cheese and in the fresh or frozen storage of meat, and in innumerable
other moist food products during holding in air.
Drying
of foods implies the removal of water from the foodstuff. In most cases,
drying is accomplished by vaporizing the water that is contained in the
food, and to do this the latent heat of vaporization must be supplied.
There are, thus, two important process-controlling factors that enter
into the unit operation of drying:
(a)
transfer of heat to provide the necessary latent heat of vaporization,
(b) movement of water or water vapour through the food material and then
away from it to effect separation of water from foodstuff.
Drying processes
fall into three categories:
 Air and contact drying under atmospheric pressure. In air and contact
drying, heat is transferred through the foodstuff either from heated air
or from heated surfaces. The water vapour is removed with the air.
Air and contact drying under atmospheric pressure. In air and contact
drying, heat is transferred through the foodstuff either from heated air
or from heated surfaces. The water vapour is removed with the air.
 Vacuum drying.
In vacuum drying, advantage is taken of the fact that evaporation of water
occurs more readily at lower pressures than at higher ones. Heat transfer
in vacuum drying is generally by conduction, sometimes by radiation. Vacuum drying.
In vacuum drying, advantage is taken of the fact that evaporation of water
occurs more readily at lower pressures than at higher ones. Heat transfer
in vacuum drying is generally by conduction, sometimes by radiation.
 Freeze drying.
In freeze drying, the water vapour is sublimed off frozen food. The food
structure is better maintained under these conditions. Suitable temperatures
and pressures must be established in the dryer to ensure that sublimation
occurs. Freeze drying.
In freeze drying, the water vapour is sublimed off frozen food. The food
structure is better maintained under these conditions. Suitable temperatures
and pressures must be established in the dryer to ensure that sublimation
occurs.
BASIC DRYING
THEORY
Three States of Water
Pure water can exist in three states, solid, liquid and vapour.
The state in which it is at any time depends on the temperature and pressure
conditions and it is possible to illustrate this on a phase diagram,
as in Fig. 7.1.
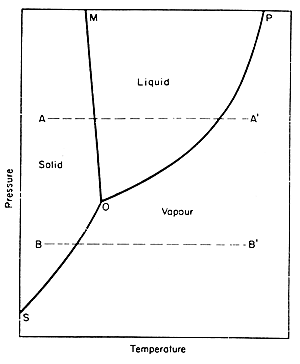
Figure 7.1 Phase diagram for water
If we choose any condition of temperature and pressure and find the corresponding
point on the diagram, this point will lie, in general, in one of the three
labelled regions, solid, liquid, or gas. This will give the state of the
water under the chosen conditions.
Under
certain conditions, two states may exist side by side, and such conditions
are found only along the lines of the diagram. Under one condition, all
three states may exist together; this condition arises at what is called
the triple point, indicated by point O on the diagram. For water it occurs
at 0.0098°C and 0.64 kPa (4.8 mm of mercury) pressure.
If
heat is applied to water in any state at constant pressure, the temperature
rises and the condition moves horizontally across the diagram, and as
it crosses the boundaries a change of state will occur. For example, starting
from condition A on the diagram adding heat warms the ice, then melts
it, then warms the water and finally evaporates the water to condition
A'. Starting from condition B, situated below the triple point, when heat
is added, the ice warms and then sublimes without passing through any
liquid state.
Liquid
and vapour coexist in equilibrium only under the conditions along the
line OP. This line is called the vapour pressure/temperature line. The
vapour pressure is the measure of the tendency of molecules to escape
as a gas from the liquid. The vapour pressure/temperature curve
for water is shown in Fig. 7.2, which is just an enlargement for
water of the curve OP of Fig. 7.1.
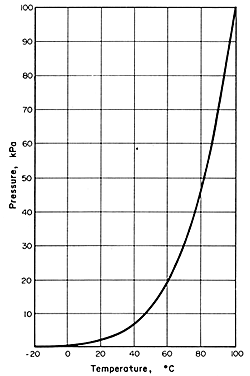
Figure 7.2. Vapour pressure/temperature curve for water
Boiling
occurs when the vapour pressure of the water is equal to the total pressure
on the water surface. The boiling point at atmospheric pressure is of
course 100°C. At pressures above or below atmospheric, water boils
at the corresponding temperatures above or below 100°C, as shown in
Fig. 7.2 for temperatures below 100°C.
Heat Requirements for Vaporization
The energy, which must be supplied to vaporize the water at any temperature,
depends upon this temperature. The quantity of energy required per kg
of water is called the latent heat of vaporization, if it is from
a liquid, or latent heat of sublimation if it is from a solid.
The heat energy required to vaporize water under any given set of conditions
can be calculated from the latent heats given in the steam table in Appendix
8, as steam and water vapour are the same thing.
 EXAMPLE
7.1. Heat energy in air drying EXAMPLE
7.1. Heat energy in air drying
A food containing 80% water is to be dried at 100°C down to moisture
content of 10%. If the initial temperature of the food is 21°C, calculate
the quantity of heat energy required per unit weight of the original material,
for drying under atmospheric pressure. The latent heat of vaporization
of water at 100°C and at standard atmospheric pressure is 2257 kJ
kg-1. The specific heat capacity of the food is 3.8 kJ kg-1
°C-1 and of water is 4.186 kJ kg-1 °C-1.
Find also the energy requirement/kg water removed.
Calculating for
1 kg food
Initial moisture = 80%
800 g moisture are associated with 200 g dry matter.
Final moisture = 10 %,
100 g moisture are associated with 900 g dry matter,
Therefore (100 x 200)/900 g = 22.2 g moisture are associated with 200
g dry matter.
1kg of original matter must lose (800 - 22) g moisture = 778 g = 0.778
kg moisture.
Heat energy required
for 1kg original material
= heat energy to raise temperature to 100°C + latent heat to remove
water
= (100 - 21) x 3.8 + 0.778 x 2257
= 300.2
+ 1755.9
= 2056 kJ.
Energy/kg water removed,
as 2056 kJ are required to remove 0.778 kg of water,
= 2056/0.778
= 2643 kJ.
Steam
is often used to supply heat to air or to surfaces used for drying. In
condensing, steam gives up its latent heat of vaporization; in drying,
the substance being dried must take up latent heat of vaporization to
convert its liquid into vapour, so it might be reasoned that 1 kg of steam
condensing will produce 1 kg vapour. This is not exactly true, as the
steam and the food will in general be under different pressures with the
food at the lower pressure. Latent heats of vaporization are slightly
higher at lower pressures, as shown in Table 7.1. In practice,
there are also heat losses and sensible heat changes which may require
to be considered.
TABLE 7.1
LATENT HEAT
AND SATURATION TEMPERATURE OF WATER
|
Absolute
pressure
|
Latent
heat of vaporization
|
Saturation
temperature
|
|
(kPa)
|
(kJ
kg-1)
|
(°C)
|
|
|
2485
|
7
|
|
2
|
2460
|
18
|
|
5
|
2424
|
33
|
|
10
|
2393
|
46
|
|
20
|
2358
|
60
|
|
50
|
2305
|
81
|
|
100
|
2258
|
99.6
|
|
101.35
(1 atm)
|
2257
|
100
|
|
110
|
2251
|
102
|
|
120
|
2244
|
105
|
|
200
|
2202
|
120
|
|
500
|
2109
|
152
|
 EXAMPLE
7.2. Heat energy in vacuum drying EXAMPLE
7.2. Heat energy in vacuum drying
Using the same material as in Example 7.1, if vacuum drying is to be carried
out at 60°C under the corresponding saturation pressure of 20 kPa
abs. (or a vacuum of 81.4 kPa), calculate the heat energy required to
remove the moisture per unit weight of raw material.
Heat energy required
per kg raw material
= heat energy to raise temperature to 60°C + latent heat of vaporization
at 20 kPa abs.
= (60 - 21) x 3.8 + 0.778 x 2358
= 148.2 + 1834.5
= 1983 kJ.
In freeze drying the latent heat of sublimation must be supplied. Pressure
has little effect on the latent heat of sublimation, which can be taken
as 2838 kJ kg-1.
 EXAMPLE
7.3. Heat energy in freeze drying EXAMPLE
7.3. Heat energy in freeze drying
If the foodstuff in the two previous examples were to be freeze dried
at 0°C, how much energy would be required per kg of raw material,
starting from frozen food at 0°C?
Heat energy required
per kilogram of raw material= latent heat of sublimation
= 0.778 x 2838
=
2208 kJ.
Heat Transfer in Drying
We have been discussing the heat energy requirements for the drying process.
The rates of drying are generally determined by the rates at which heat
energy can be transferred to the water or to the ice in order to provide
the latent heats, though under some circumstances the rate of mass transfer
(removal of the water) can be limiting. All three of the mechanisms by
which heat is transferred - conduction, radiation and convection - may
enter into drying. The relative importance of the mechanisms varies from
one drying process to another and very often one mode of heat transfer
predominates to such an extent that it governs the overall process.
As an example, in
air drying the rate of heat transfer is given by:
q = hsA(Ta
- Ts)
(7.1)
where
q is the heat transfer rate in J s-1, hs
is the surface heat-transfer coefficient J m-2 s-1 °C-1,
A is the area through which heat flow is taking place, m2,
Ta is the air temperature and Ts is
the temperature of the surface which is drying, °C.
To
take another example, in a roller dryer where moist material is spread
over the surface of a heated drum, heat transfer occurs by conduction
from the drum to the foodstuff, so that the equation is
q = UA(Ti– Ts )
where U is the overall heat-transfer coefficient, Ti
is the drum temperature (usually very close to that of the steam), Ts
is the surface temperature of the food (boiling point of water or slightly
above) and A is the area of drying surface on the drum.
The
value of U can be estimated from the conductivity of the drum material
and of the layer of foodstuff. Values of U have been quoted as
high as 1800 J m-2 s-1 °C-1 under
very good conditions and down to about 60 J m-2 s-1 °C-1
under poor conditions.
In
cases where substantial quantities of heat are transferred by radiation,
it should be remembered that the surface temperature of the food may be
higher than the air temperature. Estimates of surface temperature can
be made using the relationships developed for radiant heat transfer although
the actual effect of combined radiation and evaporative cooling is complex.
Convection coefficients also can be estimated using the standard equations.
For
freeze drying, energy must be transferred to the surface at which sublimation
occurs. However, it must be supplied at such a rate as not to increase
the temperature at the drying surface above the freezing point. In many
applications of freeze drying, the heat transfer occurs mainly by conduction.
As
drying proceeds, the character of the heat transfer situation changes.
Dry material begins to occupy the surface layers and conduction must take
place through these dry surface layers which are poor heat conductors
so that heat is transferred to the drying region progressively more slowly.
Dryer Efficiencies
Energy
efficiency in drying is of obvious importance as energy consumption is
such a large component of drying costs. Basically it is a simple ratio
of the minimum energy needed to the energy actually consumed. But because
of the complex relationships of the food, the water, and the drying medium
which is often air, a number of efficiency measures can be worked out,
each appropriate to circumstances and therefore selectable to bring out
special features important in the particular process. Efficiency calculations
are useful when assessing the performance of a dryer, looking for improvements,
and in making comparisons between the various classes of dryers which
may be alternatives for a particular drying operation.
Heat
has to be supplied to separate the water from the food. The minimum quantity
of heat that will remove the required water is that needed to supply the
latent heat of evaporation, so one measure of efficiency is the ratio
of that minimum to the energy actually provided for the process. Sensible
heat can also be added to the minimum, as this added heat in the food
often cannot be economically recovered.
Yet
another useful measure for air drying such as in spray dryers, is to look
at a heat balance over the air, treating the dryer as adiabatic with no
exchange of heat with the surroundings. Then the useful heat transferred
to the food for its drying corresponds to the drop in temperature in the
drying air, and the heat which has to be supplied corresponds to the rise
of temperature of the air in the air heater. So this adiabatic air-drying
efficiency, h,
can be defined by:
h
= (T1
- T2)/(T1 - Ta)
(7.2)
where
T1 is the inlet (high) air temperature into the dryer,
T2 is the outlet air temperature from the dryer, and
Ta is the ambient air temperature. The numerator, the
gap between T1 and T2, is a major
factor in the efficiency.
 EXAMPLE
7.4. Efficiency of a potato dryer EXAMPLE
7.4. Efficiency of a potato dryer
A dryer reduces the moisture content of 100 kg of a potato product from
80% to 10% moisture. 250 kg of steam at 70 kPa gauge is used to heat 49,800
m3 of air to 80°C, and the air is cooled to 71°C in
passing through the dryer. Calculate the efficiency of the dryer. The
specific heat of potato is 3.43 kJ kg-1 °C-1.
Assume potato enters at 24°C, which is also the ambient air temperature,
and leaves at the same temperature as the exit air.
In 100 kg of raw
material there is 80% moisture, that is 80 kg water and 20 kg dry material,
total weight of dry product = 20 x (10/9)
= 22.2 kg
weight of water = (22.2 - 20)
= 2.2 kg.
water removed = (80 - 2.2)
= 77.8 kg.
Heat supplied to
potato product
= sensible heat to raise potato product temperature from 24°C to 71°C
+ latent heat of vaporization.
Now, the latent
heat of vaporization corresponding to a saturation temperature of 71°C
is 2331 kJ kg-1
Heat (minimum) supplied/100 kg potato
= 100 x (71 - 24) x 3.43 + 77.8 x 2331
= 16 x 103 + 181 x 103
= 1.97 x 105 kJ.
Heat to evaporate water only = 77.8 x 2331
= 1.81 x 105 kJ
The specific heat
of air is 1.0 J kg-1 °C-1 and the density of
air is 1.06 kg m-3 (Appendix 3)
Heat given up by air/100 kg
potato
= 1.0 x (80 - 71) x 49,800 x 1.06
= 4.75 x 105 kJ.
The latent heat of
steam at 70 kPa gauge is 2283 kJ kg-1
Heat in steam = 250 x 2283
= 5.71 x 105 kJ.
Therefore (a) efficiency
based on latent heat of vaporisation only:
= (1.81 x 105)/ (5.71 x 105)
= 32%
(b)
efficiency assuming sensible heat remaining in food after drying is unavailable
= (1.97 x 105)/ (5.71 x 105)
= 36%
(c) efficiency based heat input and output, in drying air
= (80 – 71)/ (80 – 24)
= 16%
Whichever
of these is chosen depends on the objective for considering efficiency.
For example in a spray dryer, the efficiency calculated on the air temperatures
shows clearly and emphatically the advantages gained by operating at the
highest feasible air inlet temperature and the lowest air outlet temperatures
that can be employed in the dryer.
Examples of overall
thermal efficiencies are:
drum dryers 35-80%
spray dryers 20-50%
radiant dryers 30-40%
After
sufficient energy has been provided to vaporize or to sublime moisture
from the food, some way must be found to remove this moisture. In freeze-drying
and vacuum systems it is normally convenient to condense the water to
a liquid or a solid and then the vacuum pumps have to handle only the
non-condensible gases. In atmospheric drying a current of air is normally
used.
 Drying
> MASS TRANSFER IN DRYING Drying
> MASS TRANSFER IN DRYING
 Back
to the top Back
to the top |
![]() Basic
Drying Theory
Basic
Drying Theory![]() Three
States of Water
Three
States of Water ![]() Heat
Requirements for Vaporization
Heat
Requirements for Vaporization![]() Heat
Transfer in Drying
Heat
Transfer in Drying![]() Dryer
Efficiencies
Dryer
Efficiencies

