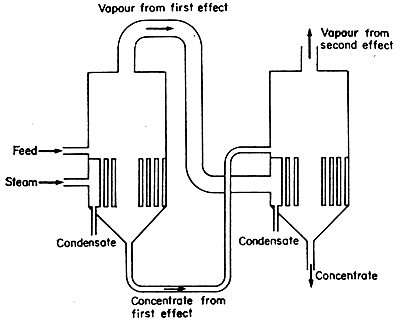
Figure 8.2 Double effect evaporator – forward feed
If
liquid is to be evaporated in each effect, and if the boiling point of
this liquid is unaffected by the solute concentration, then writing a
heat balance for the first evaporator:
q1
= U1A1(Ts - T1)
= U1A1 DT1
(8.1)
where
q1 is the rate of heat transfer, U1
is the overall heat transfer coefficient in evaporator 1, A1
is the heat-transfer area in evaporator 1, Ts is the
temperature of condensing steam from the boiler, T1
is the boiling temperature of the liquid in evaporator 1 and DT1
is the temperature difference in evaporator 1, = (Ts
- T1).
Similarly,
in the second evaporator, remembering that the "steam" in the
second is the vapour from the first evaporator and that this will condense
at approximately the same temperature as it boiled, since pressure changes
are small,
q2
= U2A2(T1 - T2)
= U2A2 DT2
in which the subscripts
2 indicate the conditions in the second evaporator.
If
the evaporators are working in balance, then all of the vapours from the
first effect are condensing and in their turn evaporating vapours in the
second effect. Also assuming that heat losses can be neglected, there
is no appreciable boiling-point elevation of the more concentrated solution,
and the feed is supplied at its boiling point,
q1
= q2
Further, if the evaporators
are so constructed that A1 = A2, the
foregoing equations can be combined.
U2/U1
= DT1/DT2.
(8.2)
Equation
(8.2) states that the temperature differences are inversely proportional
to the overall heat transfer coefficients in the two effects. This analysis
may be extended to any number of effects operated in series, in the same
way.
Feeding of Multiple Effect Evaporators
In a two effect evaporator, the temperature in the steam chest is higher
in the first than in the second effect. In order that the steam provided
by the evaporation in the first effect will boil off liquid in the second
effect, the boiling temperature in the second effect must be lower and
so that effect must be under lower pressure.
Consequently,
the pressure in the second effect must be reduced below that in the first.
In some cases, the first effect may be at a pressure above atmospheric;
or the first effect may be at atmospheric pressure and the second and
subsequent effects have therefore to be under increasingly lower pressures.
Often many of the later effects are under vacuum. Under these conditions,
the liquid feed progress is simplest if it passes from effect one to effect
two, to effect three, and so on, as in these circumstances the feed will
flow without pumping. This is called forward feed. It
means that the most concentrated liquids will occur in the last effect.
Alternatively, feed may pass in the reverse direction, starting in the
last effect and proceeding to the first, but in this case the liquid has
to be pumped from one effect to the next against the pressure drops. This
is called backward feed and because the concentrated
viscous liquids can be handled at the highest temperatures in the first
effects it usually offers larger evaporation capacity than forward feed
systems, but it may be disadvantageous from the viewpoint of product quality.
Advantages of Multiple Effect Evaporators
At first sight, it may seem that the multiple effect evaporator has all
the advantages, the heat is used over and over again and we appear to
be getting the evaporation in the second and subsequent effects for nothing
in terms of energy costs. Closer examination shows, however, that there
is a price to be paid for the heat economy.
In
the first effect, q1 = U1A1DT1
and in the second effect, q2 = U2A2DT2.
We
shall now consider a single-effect evaporator, working under the same
pressure as the first effect
qs = UsAsDTs,
where subscript s indicates the single-effect evaporator.
Since
the overall conditions are the same, DTs
= DT1+
DT2,
as the overall temperature drop is between the steam-condensing temperature
in the first effect and the evaporating temperature in the second effect.
Each successive steam chest in the multiple-effect evaporator condenses
at the same temperature as that at which the previous effect is evaporating.
Now,
consider the case in which U1 = U2
= Us, and A1 = A2.
The problem then becomes to find As for the single-effect
evaporator that will evaporate the same quantity as the two effects.
From the given conditions
and from eqn. (8.2),
DT1
= DT2
and DTs=
DT1
+ DT2
= 2DT1
DT1
= 0.5DTs
Now q1 + q2 = U1A1DT1
+ U2A2DT2
= U1(A1+
A2) DTs/2
but q1
+ q2 = qs
and qs
= UAsDTs
so that (A1
+ A2)/2 = 2A1/2 = As
That is A1 = A2 = As
The
analysis shows that if the same total quantity is to be evaporated, then
the heat transfer surface of each of the two effects must be the same
as that for a single effect evaporator working between the same overall
conditions. The analysis can be extended to cover any number of effects
and leads to the same conclusions. In multiple effect evaporators, steam
economy has to be paid for by increased capital costs of the evaporators.
Since the heat transfer areas are generally equal in the various effects
and since in a sense what you are buying in an evaporator is suitable
heat transfer surface, the n effects will cost approximately
n times as much as a single effect.
Comparative
costs of the auxiliary equipment do not altogether follow the same pattern.
Condenser requirements are less for multiple effect evaporators. The condensation
duty is distributed between the steam chests of the effects, except for
the first one, and so condenser and cooling water requirements will be
less. The optimum design of evaporation plant must then be based on a
balance between operating costs which are lower for multiple effects because
of their reduced steam consumption, and capital charges which will be
lower for fewer evaporators. The comparative operating costs are illustrated
by the figures in Table 8.1 based on data from Grosse
and Duffield (1954); if the capital costs were available they would
reduce the advantages of the multiple effects, but certainly not remove
them.
TABLE 8.1
STEAM CONSUMPTION AND RUNNING COSTS OF EVAPORATORS
| Number of
effects |
Steam
consumption
(kg steam/kg water evaporated)
|
Total
running cost
(relative to a single- effect evaporator)
|
| One |
1.1
|
1
|
| Two |
0.57
|
0.52
|
| Three |
0.40
|
0.37
|
 EXAMPLE
8.4. Triple effect evaporators: steam usage and heat transfer surface EXAMPLE
8.4. Triple effect evaporators: steam usage and heat transfer surface
Estimate the requirements of steam and heat transfer surface, and the
evaporating temperatures in each effect, for a triple effect evaporator
evaporating 500 kg h-1 of a 10% solution up to a 30% solution.
Steam is available at 200 kPa gauge and the pressure in the evaporation
space in the final effect is 60 kPa absolute. Assume that the overall
heat transfer coefficients are 2270, 2000 and 1420 J m-2 s-1
°C-1 in the first, second and third effects respectively.
Neglect sensible heat effects and assume no boiling-point elevation, and
assume equal heat transfer in each effect.
Mass balance
(kg h-1)
 |
|
Solids
|
Liquids
|
Total
|
| |
Feed |
50
|
450
|
500
|
| |
Product |
50
|
117
|
167
|
| |
Evaporation |
|
|
333
|
Heat
balance
From
steam tables, the condensing temperature of steam at 200 kPa (g) is 134°C
and the latent heat is 2164 kJ kg -1. Evaporating temperature
in final effect under pressure of 60 kPa (abs.) is 86°C, as there
is no boiling-point rise and latent heat is 2294 kJ kg-1.
Equating the heat
transfer in each effect:
q1 = q2
= q3
U1A1DT1
= U2A2 DT2
= U3A3DT3
And DT1
+ DT2
+ DT3
= (134 - 86) = 48°C.
Now, if A1 = A2 = A3
then DT2
= U1DT1
/U2 and DT3
= U1DT1
/U3
so that DT1(1
+ U1/U2 + U1/U3)
= 48,
DT1
x [1 + (2270/2000) + (2270/1420)] = 48
3.73DT1
= 48
DT1
= 12.9°C,
DT2
= DT1
x (2270/2000) = 14.6°C
and DT3
= DT1
x (2270/1420) = 20.6°C
And so the evaporating
temperature:
 in first effect is
(134 - 12.9) = 121°C; latent heat (from Steam Tables) 2200 kJ kg-1.
in first effect is
(134 - 12.9) = 121°C; latent heat (from Steam Tables) 2200 kJ kg-1.
 in second effect is (121 - 14.6) = 106.5°C;
latent heat 2240 kJ kg-1
in second effect is (121 - 14.6) = 106.5°C;
latent heat 2240 kJ kg-1
 in the third effect is (106.5 - 20.6) = 86°C, latent heat 2294 kJ
kg-1
in the third effect is (106.5 - 20.6) = 86°C, latent heat 2294 kJ
kg-1
Equating the quantities
evaporated in each effect and neglecting the sensible heat changes, if
w1, w2, w3 are the respective quantities
evaporated in effects 1,2 and 3, and ws is the quantity
of steam condensed per hour in effect 1, then
w1 x 2200 x 103 = w2 x 2240
x 103
= w3 x 2294 x 103
= ws x 2164 x 103
The
sum of the quantities evaporated in each effect must equal the total evaporated
in all three effects so that:
w1
+ w2 + w3 = 333 and solving as above,
w1 = 113 kg h-1
w2 = 111kg h-1 w3
= 108kg h-1
ws
= 115 kg h-1
Steam consumption
It
required 115 kg steam (ws) to evaporate a total of 333
kg water, that is
0.35kg steam/kg water evaporated.
Heat exchanger
surface.
Writing
a heat balance on the first effect:
(113 x 2200 x 1000)/3600
= 2270 x A1 x 12.9
A1 = 2.4 m2 = A2 = A3
total area = A1 + A2
+ A3 = 7.2 m2.
Note
that the conditions of this example are considerably simplified, in that
sensible heat and feed heating effects are neglected, and no boiling-point
rise occurs. The general method remains the same in the more complicated
cases, but it is often easier to solve the heat balance equations by trial
and error rather than by analytical methods, refining the approximations
as far as necessary.
 Evaporation
> VAPOUR RECOMPRESSION Evaporation
> VAPOUR RECOMPRESSION
 Back
to the top Back
to the top |
![]() Feeding
of Multiple Effect Evaporators
Feeding
of Multiple Effect Evaporators![]() Advantages
of Multiple Effect Evaporators
Advantages
of Multiple Effect Evaporators 
