In
studying chilling and freezing, it is necessary to look first at the methods
for obtaining low temperatures, i.e. refrigeration systems, and then at
the coupling of these to the food products in chilling and freezing.
Refrigeration Cycle
The basis of mechanical refrigeration is the fact that at different pressures
the saturation (or the condensing) temperatures of vapours are different
as clearly shown on the appropriate vapour-pressure/temperature curve.
As the pressure increases condensing temperatures also increase. This
fact is applied in a cyclic process, which is illustrated in Fig.
6.8.
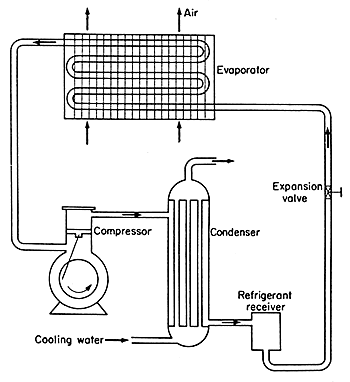
Figure 6.8 Mechanical refrigeration circuit
The process can be followed on the pressure-enthalpy (P/H)
chart shown on Fig. 6.9.
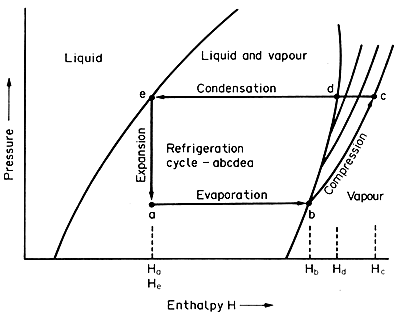
Figure 6.9 Pressure/enthalpy chart
This is a thermodynamic diagram that looks very complicated at first sight
but which in fact can make calculations straightforward and simple. For
the present purposes the most convenient such chart is the one shown with
pressure as the vertical axis (for convenience on a logarithmic scale)
and enthalpy on the horizontal axis. On such a diagram, the properties
of the particular refrigerant can be plotted, including the interphase
equilibrium lines such as the saturated vapour line, which are important
as refrigeration depends on evaporation and condensation. Figure 6.9 is
a skeleton diagram and Appendix 11 gives
charts for two common refrigerants, refrigerant 134a, tetrafluoroethane
(in Appendix 11a), and ammonia (in 11b); others for common refrigerants
can be found in the ASHRAE Guide and Data Books.
To
start with the evaporator; in this the pressure above the refrigerant
is low enough so that evaporation of the refrigerant liquid to a gas occurs
at some suitable low temperature determined by the requirements of the
product. This temperature might be, for example -18°C in which case
the corresponding pressures would be for ammonia 229 kPa absolute and
for tetrafluoroethane (also known as refrigerant 134a) 144 kPa. Evaporation
then occurs and this extracts the latent heat of vaporization for the
refrigerant from the surroundings of the evaporator and it does this at
the appropriate low temperature. This process of heat extraction at low
temperature represents the useful part of the refrigerator. On the pressure/enthalpy
chart this is represented by ab at constant pressure (the evaporation
pressure) in which 1 kg of refrigerant takes in (Hb
- Ha) kJ . The low pressure necessary for the evaporation
at the required temperature is maintained by the suction of the compressor.
The
remainder of the process cycle is included merely so that the refrigerant
may be returned to the evaporator to continue the cycle. First, the vapour
is sucked into a compressor which is essentially a gas pump and which
increases its pressure to exhaust it at the higher pressure to the condensers.
This is represented by the line bc which follows an adiabatic
compression line, a line of constant entropy (the reasons for this
must be sought in a book on refrigeration) and work equivalent to (Hc
- Hb) kJ kg-1 has to be performed on the
refrigerant to effect the compression. The higher pressure might be, for
example, 1150 kPa (pressures are absolute pressures) for ammonia, or 772
kPa for refrigerant 134a, and it is determined by the temperature at which
cooling water or air is available to cool the condensers.
To
complete the cycle, the refrigerant must be condensed, giving up its latent
heat of vaporization to some cooling medium. This is carried out in a
condenser, which is a heat exchanger cooled generally by water or air.
Condensation is shown on Fig. 6.9 along the horizontal line (at the constant
condenser pressure), at first cd cooling the gas and then continuing
along de until the refrigerant is completely condensed at point
e. The total heat given out in this from refrigerant to condenser
water is (Hc - He) = (Hc
- Ha) kJ kg-1. The condensing temperature,
corresponding to the above high pressures, is about 30°C and so in
this example cooling water at about 20°C, could be used, leaving sufficient
temperature difference to accomplish the heat exchange in equipment of
economic size.
This
process of evaporation at a low pressure and corresponding low temperature,
followed by compression, followed by condensation at around atmospheric
temperature and corresponding high pressure, is the refrigeration cycle.
The high-pressure liquid then passes through a nozzle from the condenser
or high-pressure receiver vessel to the evaporator at low pressure, and
so the cycle continues. Expansion through the expansion valve nozzle is
at constant enthalpy and so it follows the vertical line ea with
no enthalpy added to or subtracted from the refrigerant. This line at
constant enthalpy from point e explains why the point a
is where it is on the pressure line, corresponding to the evaporation
(suction) pressure.
By
adjusting the high and low pressures, the condensing and evaporating temperatures
can be selected as required. The high pressure is determined: by the available
cooling-water temperature, by the cost of this cooling water and by the
cost of condensing equipment. The evaporating pressure is determined by
either the low temperature that is required for the product or by the
rate of cooling or freezing that has to be provided. Low evaporating temperatures
mean higher power requirements for compression and greater volumes of
low-pressure vapours to be handled therefore larger compressors, so that
the compression is more expensive. It must also be remembered that, in
actual operation, temperature differences must be provided to operate
both the evaporator and the condenser. There must be lower pressures than
those that correspond to the evaporating coil temperature in the compressor
suction line, and higher pressures in the compressor discharge than those
that correspond to the condenser temperature.
Overall,
the energy side of the refrigeration cycle can therefore be summed up:
 heat taken in from surroundings at the (low) evaporator temperature and
pressure (Hb - Ha),
heat taken in from surroundings at the (low) evaporator temperature and
pressure (Hb - Ha),
 heat equivalent to the work done by the compressor (Hc
- Hb) and
heat equivalent to the work done by the compressor (Hc
- Hb) and
 heat rejected at the (high) compressor pressure and temperature (Hc
- He).
heat rejected at the (high) compressor pressure and temperature (Hc
- He).
A
useful measure is the ratio of the heat taken in at the evaporator (the
useful refrigeration), (Hb - Ha) ,
to the energy put in by the compressor which must be paid for (Hc–
Hb). This ratio is called the coefficient of performance
(COP).
The unit commonly used to measure refrigerating effect is the ton
of refrigeration = 3.52 kW. It arises from the quantity of energy
to freeze 2000 lb of water in one day (2000 lb is called 1 short ton).
 EXAMPLE
6.7. Freezing of fish EXAMPLE
6.7. Freezing of fish
It is wished to freeze 15 tonnes of fish per day from an initial temperature
of 10°C to a final temperature of -8°C using a stream of cold
air. Estimate the maximum capacity of the refrigeration plant required,
if it is assumed that the maximum rate of heat extraction from the product
is twice the average rate. If the heat-transfer coefficient from the air
to the evaporator coils, which form the heat exchanger between the air
and the boiling refrigerant, is 22 J m-2 s-1 °C-1,
calculate the surface area of evaporator coil required if the logarithmic
mean temperature drop across the coil is 12°C.
From
the tabulated data (Appendix 7)
the specific heat of fish is 3.18 kJ kg-1 °C-1
above freezing and 1.67 kJ kg-1 °C-1 below freezing,
and the latent heat is 276 kJ/kg-1.
Enthalpy change in fish: = heat loss above freezing + heat loss below
freezing + latent heat
= (10 x 3.18) + (8 x 1.67)
+ 276 = 31.8 + 13.4 + 276
= 321.2 kJ kg-1
Total heat removed in freezing:
15 x 1000 x 321.2 = 4.82 x 106
kJ day-1
Average rate of heat removal:
(4.82 x 106)/(24
x 60 x 60) = 55.8 kJ s-1
If maximum is twice
average
then
maximum = q = 111.6 kJ s-1
= 111.6 x 103 J s-1
Coil rate of heat
transfer
q = UADTm
and so
A = q/UDTm
= (111.6 x 103)/(22 x 12)
= 0.42
x 103 m2
= 420
m2.
The
great advantage of tracing the cycle on the pressure/enthalpy diagram
is that from the numerical coordinates of the various cycle points performance
parameters can be read off or calculated readily.
 EXAMPLE
6.8. Rate of boiling of refrigerant EXAMPLE
6.8. Rate of boiling of refrigerant
Ammonia liquid is boiling in an evaporator under an absolute pressure
of 120 kPa. Find the temperature and the volumetric rate of evolution
of ammonia gas if the heat extraction rate from the surroundings is 300
watts.
From the chart in
Appendix 11(b) the boiling temperature of
ammonia at a pressure of 120 kPa is -30°C, so this is the evaporator
temperature.
Also from the chart,
the latent heat of evaporation is:
(enthalpy of saturated vapour - enthalpy of saturated liquid)
= 1.72
- 0.36 = 1.36 MJ kg-1 = 1.36 x 103kJ kg-1
For a heat-removal
rate of 300 watts = 0.3 kJ s-1 the ammonia evaporation rate
is:
(0.3/1360)
= 2.2 x 10-4 kg s-1.
The
chart shows at the saturated vapour point for the cycle (b on Fig. 6.8)
that the specific volume of ammonia vapour is:
0.98
m3 kg-1
and so the volumetric rate of ammonia evolution is:
2.2 x 10-4 x 0.98 = 2.16 x10-4 m3
s-1.
So
far we have been talking of the theoretical cycle. Real cycles differ
by, for example, pressure drops in piping, superheating of vapour to the
compressor and non-adiabatic compression, but these are relatively minor.
Approximations quite good enough for our purposes can be based on the
theoretical cycle. If necessary, allowances can be included for particular
inefficiencies.
The
refrigerant vapour has to be compressed so it can continue round the cycle
and be condensed. From the refrigeration demand, the weight of refrigerant
required to be circulated can be calculated; each kg s-1 extracts
so many J s-1 according to the value of (Hb
- Ha). From the volume of this refrigerant, the compressor
displacement can be calculated, as the compressor has to handle this volume.
Because the compressor piston cannot entirely displace all of the working
volume of the cylinder, there must be a clearance volume; and because
of inefficiencies in valves and ports, the actual amount of refrigerant
vapour taken in is less than the theoretical. The ratio of these, actual
amount taken in, to the theoretical compressor displacement, is called
the volumetric efficiency of the compressor. Both mechanical
and volumetric efficiencies can be measured, or taken from manufacturer's
data, and they depend on the actual detail of the equipment used.
Consideration
of the data from the thermo-dynamic chart and of the refrigeration cycle
enables quite extensive calculations to be made about the operation.
 EXAMPLE
6.9. Operation of a compressor in a refrigeration system EXAMPLE
6.9. Operation of a compressor in a refrigeration system
To meet the requirements of Example 6.7, calculate the speed at which
it would be necessary to run a six-cylinder reciprocating ammonia compressor
with each cylinder having a 10-cm bore (diameter) and a 12-cm stroke (length
of piston travel), assuming a volumetric efficiency of 80%. The condensing
temperature is 30°C (determined from the available cooling water temperature)
and the evaporating temperature needed is -15°C. Calculate also the
theoretical coefficient of performance of this refrigeration system.
From the chart Appendix
11(b), the heat extracted by ammonia at the evaporating temperature
of -15°C is (1.74-0.63) = 1.11 MJ kg-1 = 1.11x 103J kg-1
Maximum rate of
refrigeration
= 111.6 kJ s-1
Rate of refrigerant circulation = 111.6/1110 = 0.100 kg s-1
Specific volume of refrigerant (from chart) = 0.49
m3 kg-1
Theoretical displacement volume
= 0.49 x 0.100 = 0.049 m3 s-1
Actual displacement needed
= (0.049 x 100)/80
m3 s-1 = 61.3 x 103m3 s-1
Volume of cylinders
= (p/4)
x (0.10)2 x 0.12 x 6
= 5.7 x 10-3 m3 swept out per rev.
Speed of compressor
= (61.3 x 10-3)/(5.7 x 10-3)
= 10.8 rev s-1 = 645 rev. min-1
Coefficient of performance =
(heat energy extracted in evaporator)/(heat equivalent of theoretical
energy input in the compressor)
= (Hb - Ha)/(Hc
- Hb)
Under cycle conditions,
from chart, the coefficient of performance
= (1.74- 0.63)/(1.97 -1.74)
= 1.11/0.23
= 4.8.
Performance Characteristics
Variations in load and in the evaporating or condensing temperatures are
often encountered when considering refrigeration systems. Their effects
can be predicted by relating them to the basic cycle.
If
the heat load increases in the cold store, then the temperature tends
to rise and this increases the amount of refrigerant boiling off. If the
compressor cannot move this, then the pressure on the suction side of
the compressor increases and so the evaporating temperature increases
tending to reduce the evaporation rate and correct the situation. However,
the effect is to lift the temperature in the cold space and if this is
to be prevented additional compressor capacity is required.
As
the evaporating pressure, and resultant temperature, change, so the volume
of vapour per kilogram of refrigerant changes. If the pressure decreases,
this volume increases, and therefore the refrigerating effect, which is
substantially determined by the rate of circulation of refrigerant, must
also decrease. Therefore if a compressor is required to work from a lower
suction pressure its capacity is reduced, and conversely. So at high suction
pressures giving high circulation rates, the driving motors may become
overloaded because of the substantial increase in quantity of refrigerant
circulated in unit time.
Changes
in the condenser pressure have relatively little effect on the quantity
of refrigerant circulated. However, changes in the condenser pressure
and also decreases in suction pressure, have quite a substantial effect
on the power consumed per ton of refrigeration. Therefore for an economical
plant, it is important to keep the suction pressure as high as possible,
compatible with the product requirement for low temperature or rapid freezing,
and the condenser pressure as low as possible compatible with the available
cooling water or air temperature.
Refrigerants
Although in theory a considerable number of fluids might be used in mechanical
refrigeration, and historically quite a number including cold air and
carbon dioxide have been, those actually in use today are only a very
small number. Substantially they include only ammonia, which is used in
many large industrial systems, and approved members of a family of halogenated
hydrocarbons containing differing proportions of fluorine and chosen according
to the particular refrigeration duty required. The reasons for this very
small group of practical refrigerants are many.
Important reasons are:
 actual
vapour pressure/temperature curve for the refrigerant which determines
the pressures between which the system must operate for any particular
pair of evaporator and condenser temperatures, actual
vapour pressure/temperature curve for the refrigerant which determines
the pressures between which the system must operate for any particular
pair of evaporator and condenser temperatures,
 refrigerating
effect per cubic metre of refrigerant pumped around the system which in
turn governs the size of compressors and piping, and the stability and
cost of the refrigerant itself. refrigerating
effect per cubic metre of refrigerant pumped around the system which in
turn governs the size of compressors and piping, and the stability and
cost of the refrigerant itself.
Ammonia
is in most ways the best refrigerant from the mechanical point of view,
but its great problem is its toxicity. The thermodynamic chart for ammonia,
refrigerant 717 is given in Appendix 11(b).
In working spaces, such as encountered
in air conditioning, the halogenated hydrocarbons (often known, after
the commercial name, as Freons) are very often used because of safety
considerations. They are also used in domestic refrigerators. Environmental
problems, in particular due to the effects of any chlorine derivatives
and their long lives in the upper atmosphere, have militated heavily,
in recent years, against some formerly common halogenated hydrocarbons.
Only a very restricted selection has been judged safe. A thermodynamic
chart for refrigerant 134a (the reasons for the numbering system
are obscure, ammonia being refrigerant 717), the commonest of the safe
halogenated hydrocarbon refrigerants, is given in Appendix
11(a). Other charts are available in references such as those provided
by the refrigerant manufacturers and in books such as the ASHRAE
Guide and Data Books.
Mechanical Equipment
Compressors are just basically vapour pumps and much the same types as
shown in Fig. 4.3 for liquid pumps are encountered. Their design is highly
specialized, particular problems arising from the lower density and viscosity
of vapours when compared with liquids. The earliest designs were reciprocating
machines with pistons moving horizontally or vertically, at first in large
cylinders and at modest speeds, and then increasingly at higher speeds
in smaller cylinders. An important aspect in compressor choice is the
compression ratio, being the ratio of the absolute pressure of discharge
from the compressor, to the inlet suction pressure. Reciprocating compressors
can work effectively at quite high compression ratios (up to 6 or 7 to
1). Higher overall compression ratios are best handled by putting two
or more compressors in series and so sharing the overall compression ratio
between them.
For
smaller compression ratios and for handling the large volumes of vapours
encountered at low temperatures and pressures, rotary vane compressors
are often used, and for even larger volumes, centrifugal compressors,
often with many stages, can be used. A recent popular development is the
screw compressor, analogous to a gear pump, which has considerable flexibility.
Small systems are often "hermetic", implying that the motor
and compressor are sealed into one casing with the refrigerant circulating
through both. This avoids rotating seals through which refrigerant can
leak. The familiar and very dependable units in household refrigerators
are almost universally of this type.
Refrigeration Evaporator
The evaporator is the only part of the refrigeration equipment that enters
directly into food processing operations. Heat passes from the food to
the heat-transfer medium, which may be air or liquid, thence to the evaporator
and so to the refrigerant. Thermal coupling is in some cases direct, as
in a plate freezer. In this, the food to be frozen is placed directly
on or between plates, within which the refrigerant circulates. Another
familiar example of direct thermal coupling is the chilled slab in a shop
display.
Generally,
however, the heat transfer medium is air, which moves either by forced
or natural circulation between the heat source, the food and the walls
warmed by outside air, and the heat sink which is the evaporator. Sometimes
the medium is liquid such as in the case of immersion freezing in propylene
glycol or in alcohol-water mixtures. Then there are some cases in which
the refrigerant is, in effect, the medium such as immersion or spray with
liquid nitrogen. Sometimes there is also a further intermediate heat-transfer
medium, so as to provide better control, or convenience, or safety. An
example is in some milk chillers, where the basic refrigerant is ammonia;
this cools glycol, which is pumped through a heat exchanger where it cools
the milk. A sketch of some types of evaporator system is given in Fig.
6.10.
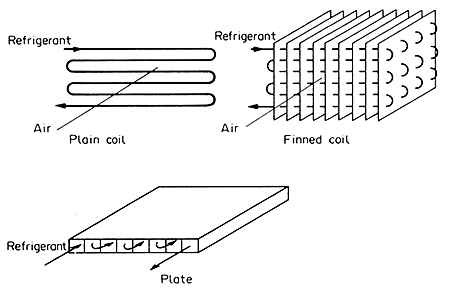
Figure 6.10 Refrigeration evaporators
In the freezer or chiller, the heat transfer rates both from the food
and to the evaporator, depend upon fluid or gas velocities and upon temperature
differences. The values for the respective heat transfer coefficients
can be estimated by use of the standard heat transfer relationships. Typical
values that occur in freezing equipment are given in Table 6.3.
TABLE 6.3
EXPECTED SURFACE HEAT TRANSFER COEFFICIENTS (hs)
| |
J
m-2 s-1 °C-1
|
|
Still-air
freezing (including radiation to coils)
|
9
|
|
Still-air
freezing (no radiation)
|
6
|
|
Air-blast
freezing 3 m s-1
|
18
|
|
Air-blast
freezing 5 m s-1
|
30
|
|
Liquid-immersion
freezing
|
600
|
|
Plate freezing
|
120
|
Temperature differences
across evaporators are generally of the order 3 - 10°C. The calculations
for heat transfer can be carried out using the methods which have been
discussed in other sections, including their relationship to freezing
and freezing times, as the evaporators are just refrigerant-to-air heat
exchangers.
The
evaporator surfaces are often extended by the use of metal fins
that are bonded to the evaporator pipe surface. The reason for this construction
is that the relatively high metal conductance, compared with the much
lower surface conductance from the metal surface to the air, maintains
the fin surface substantially at the coil temperature. A slight rise in
temperature along the fin can be accounted for by including in calculations
a fin efficiency factor. The effective evaporator area is then calculated
by the relationship
A
= Ap+ fAs
(6.5)
where
A is the equivalent total evaporator surface area, Ap
is the coil surface area, called the primary surface, As
is the fin surface area, called the secondary surface and f
(phi) is the fin efficiency.
Values of f
lie between 65% and 95% in the usual designs, as shown for example in
DKV Arbeitsblatt 2-02,
1950.
Chilling
Chilling of foods is a process by which their temperature is reduced to
the desired holding temperature just above the freezing point of food,
usually in the region of -2 to 2°C
Many
commercial chillers operate at higher temperatures, up to 10-12°C.
The effect of chilling is only to slow down deterioration changes and
the reactions are temperature dependent. So the time and temperature of
holding the chilled food determine the storage life of the food.
Rates
of chilling are governed by the laws of heat transfer which have been
described in previous sections. It is an example of unsteady-state heat
transfer by convection to the surface of the food and by conduction within
the food itself. The medium of heat exchange is generally air, which extracts
heat from the food and then gives it up to refrigerant in the evaporator.
As explained in the heat-transfer section, rates of convection heat transfer
from the surface of food and to the evaporator are much greater if the
air is in movement, being roughly proportional to v0.8.
To
calculate chilling rates it is therefore necessary to evaluate:
(a) surface heat transfer coefficient,
(b) resistance offered to heat flow by any packaging material that may
be placed round the food,
(c) appropriate unsteady state heat conduction equation.
Although
the shapes of most foodstuffs are not regular, they often approximate
the shapes of slabs, bricks, spheres and cylinders.
 EXAMPLE
6.10. Chilling of fresh apples EXAMPLE
6.10. Chilling of fresh apples
Before apples are loaded into a cool store, it is wished to chill them
to a central temperature of 5°C so as to avoid problems of putting
warm apples with the colder ones in storage. The apples, initially at
25°C, are considered to be spheres of 7 cm diameter and the chilling
is to be carried out using air at -1°C and at a velocity which provides
a surface heat-transfer coefficient of 30 J m-2 s-1
°C-1. The physical properties of the apples are k
= 0.5 J m-1 s-1 °C-1, r
= 930 kg m-3, c = 3.6 kJ kg-1 °C-1.
Calculate the time necessary to chill the apples so that their centres
reach 5°C.
This is an example
of unsteady-state cooling and can be solved by application of Fig. 5.3,
Bi = hsr/k = (30 x 0.035)/0.5 = 2.1
1/Bi = 0.48
(T–
T 0)/(T1 – T0)
= [5 – (-1)]/[25 – (-1)] = 0.23
and so, reading from Fig.5.3
Fo = 0.46 = kt/rcr2
t
= Fo rcr2
/k
so
t = [0.46x 3600 x 930 x (0.035)2]/0.5
= 3773 s
=
1.05 h
A
full analysis of chilling must, in addition to heat transfer, take mass
transfer into account if the food surfaces are moist and the air is unsaturated.
This is a common situation and complicates chilling analysis.
Freezing
Water makes up a substantial proportion of almost all foodstuffs and so
freezing has a marked physical effect on the food. Because of the presence
of substances dissolved in the water, food does not freeze at one temperature
but rather over a range of temperatures. At temperatures just below the
freezing point of water, crystals that are almost pure ice form in the
food and so the remaining solutions become more concentrated. Even at
low temperatures some water remains unfrozen, in very concentrated solutions.
In
the freezing process, added to chilling is the removal of the latent heat
of freezing. This latent heat has to be removed from any water that is
present. Since the latent heat of freezing of water is 335 kJ kg-1,
this represents the most substantial thermal quantity entering into the
process. There may be other latent heats, for example the heats of solidification
of fats which may be present, and heats of solution of salts, but these
are of smaller magnitude than the latent heat of freezing of water. Also
the fats themselves are seldom present in foods in as great a proportion
as water.
Because
of the latent-heat-removal requirement, the normal unsteady-state equations
cannot be applied to the freezing of foodstuffs. The coefficients of heat
transfer can be estimated by the following equation:
1/hs = 1/hc + (x/k) + 1/hr
where
hs is the total surface heat-transfer coefficient, hc
is the convection heat transfer coefficient, x is the thickness
of packing material, k is the thermal conductivity of the packing
material and hr is the radiation heat-transfer coefficient.
A
full analytical solution of the rate of freezing of food cannot be obtained.
However, an approximate solution, due to Plank, is sufficient for
many practical purposes. Plank assumed that the freezing process:
(a) commences with all of the food unfrozen but at its freezing temperature,
(b) occurs sufficiently slowly for heat transfer in the frozen layer to
take place under steady-state conditions.
Making
these assumptions, freezing rates for bodies of simple shapes can be calculated.
As an example of the method, the time taken to freeze to the centre
of a slab whose length and breadth are large compared with the thickness,
will be calculated.
Rates
of heat transfer are equal from either side of the slab. Assume that at
time t a thickness x of the slab of area A has been
frozen as shown in Fig. 6.11. The temperature of the
freezing medium is Ta. The freezing temperature of the
foodstuff is T, and the surface temperature of the food is Ts.
The thermal conductivity of the frozen food is k, l
is the latent heat of the foodstuff and r
is its density.
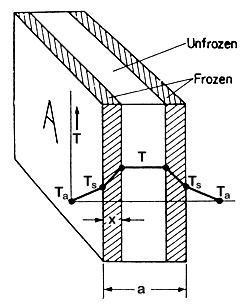
Figure 6.11 Freezing of a slab
The rate of movement of the freezing boundary multiplied by the latent
heat equals the rate of heat transfer of heat to the boundary:
q
= A lr
dx/dt
Now,
all of the heat removed at the freezing boundary must be transmitted to
the surface through the frozen layer; if the frozen layer is in the steady-state
condition we have:
q
= (T – Ts)k/x
Similarly, this quantity
of heat must be transferred to the cooling medium from the food surface,
so:
q =
Ahs(Ts - Ta)
where hs
is the surface heat-transfer coefficient.
Eliminating Ts
between the two equations gives:
q
= (T – Ta)A x 1/(1/hs
+ x/k)
Since
the same heat flow passing through the surface also passes through the
frozen layer and is removed from the water as it freezes in the centre
of the block:
A(T –Ta)/(1/hs
+ x/k) = A lr
dx/dt
Therefore (T –Ta)/(1/hs
+ x/k) = lr
dx/dt
dt(T –Ta) = lr
(1/hs + x/k)dx
Now, if the thickness of the slab is a, the time taken for the centre
of the slab at x = a/2 to freeze can be obtained by integrating
from x = 0, to x = a/2 during which time t
goes from t to tf .
Therefore tf (T –Ta)
= lr
(a/2hs + a2/8k)
And tf =
lr
(a/2hs + a2/8k)
(6.6)
(T –Ta)
In
his papers Plank (1913, 1941) derived his equation in more general terms
and found that for brick-shaped solids the change is in numerical terms,
called shape factors, only.
A general equation
can thus be written
tf =
lr
(Pa/hs + R a2/k)
(6.7)
(T –Ta)
where
P = 1/2, R = 1/8 for a slab; P = 1/4, R =
1/16 for an infinitely long cylinder, and P = 1/6, R = 1/24
for a cube or a sphere. Brick-shaped solids have values of P and
R lying between those for slabs and those for cubes. Appropriate
values of P and R for a brick-shaped solid can be obtained
from the graph in Fig. 6.12. In this figure, b1
and b2
are the ratios of the two longest sides to the shortest. It does not matter
in what order they are taken.
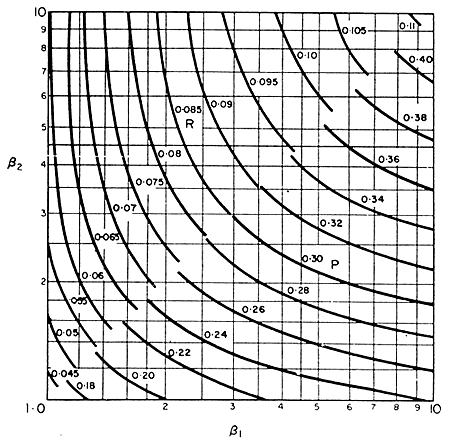
Figure 6.12 Coefficients in Plank’s Equation
Adapted from Ede, Modern Refrigeration, 1952
Because the assumptions made in the derivation of Plank's equation lead
to errors, which tend towards under-estimation of freezing times, more
accurate predictions can be made if some allowances are made for this.
One step is to use the total enthalpy changes from the initial to the
final state of the product being frozen, that is to include the sensible
heat changes both above and below the freezing temperature in addition
to the latent heat. Even with this addition the prediction will still
be about 20% or so lower than equivalent experimental measurements for
brick shapes indicate. Adaptations of Plank's equation have been proposed
which correspond better with experimental results, such as those in Cleland
and Earle (1982), but they are more complicated.
 EXAMPLE
6.11. Freezing of a slab of meat EXAMPLE
6.11. Freezing of a slab of meat
If a slab of meat is to be frozen between refrigerated plates with the
plate temperature at -34°C, how long will it take to freeze if the
slab is 10 cm thick and the meat is wrapped in cardboard 1 mm thick on
either side of the slab? What would be the freezing time if the cardboard
were not present? Assume that for the plate freezer, the surface heat-transfer
coefficient is 600 J m-2 s-1 °C-1,
the thermal conductivity of cardboard is 0.06 J m-1 s-1°
C-1 the thermal conductivity of frozen meat is 1.6 J m-1
s-1 °C-1, its latent heat is 2.56 x 105
J kg-1 and density 1090 kg m-3. Assume also that
meat freezes at -2°C.
Conductance
of cardboard packing = x/k = 0.001/0.06 = 0.017.
1/hs
= x/k + 1/hc = 0.017 + 1/600 = 0.019.
hs = 52.6 J
m-2 s-1 °C-1
In
a plate freezer, the thickness of the slab is the only dimension that
is significant. The case can be treated as equivalent to an infinite slab,
and therefore the constants in Plank's equation are 1/2 and 1/8.
tf
= lr
[Pa/hs +R a2/k]
(T –Ta)
= (2.56 x 105 x 1090) x [(0.5
x 0.1 x 0.019) +(0.125 x {0.1}2/1.6)]
(-2
- (-34))
= 1.51 x 104 s
= 4.2 h
And with no packing
hs = 600 so that
1/hs
= 1.7 x 10-3 and
tf = (2.56 x 105 x 1090)/[-2 - (-34)] x [(0.5
x 0.1 x 1.7 x 10-3) +(0.125 x {0.1}2/1.6)]
= 7.54 x 103 sec
= 2.1 h
This estimate can be improved by adding to the latent heat of freezing,
the enthalpy change above the freezing temperature and below the freezing
temperature. Above freezing, assume the meat with a specific heat of 3.22
x 103 J kg-1°C-1 starts at +10°C
and goes to -2°C, needing 3.9 x 104 J kg-1.
Below the freezing temperature assume the meat goes from -2°C to the
mean of -2°C and -34°C, that is -18°C, with a specific heat
of 1.67 x 103 J kg-1°C-1, needing
2.8 x 104 J kg-1. This, with the latent heat of
2.56 x 105 Jkg, gives a total of 3.23 x 105 J kg-1
and amended freezing times of
4.2 x (3.23/2.56) = 5.3 h
and 2.1 x (3.23/2.56) = 2.6 h.
If instead of a slab, cylinder, or cube, the food were closer to a brick
shape then an additional 20% should be added.
Thus,
with a knowledge of the thermal constants of a foodstuff, required freezing
times can be estimated by the use of Plank's equation. Appendix
7 gives values for the thermal conductivities, and the latent heats
and densities, of some common foods.
The
analysis using Plank's equation separates the total freezing time effectively
into two terms, one intrinsic to the food material to be frozen, and the
other containing the surface heat transfer coefficient which can be influenced
by the process equipment. Therefore for a sensitivity analysis, it can
be helpful to write Plank's equation in dimensionless form, substituting
DH
for lr:
tfh DT
= (1 + R Bi)
where Bi = ha = hr
aDHP
P
k k
This leads to defining
an efficiency term:
h
= RBi / (1 + R Bi) =
Bi =
Bi remembering
that P/R = 4 for a slab (6.8)
P P
P/R + Bi
4 + Bi
in
which h
(eta) can be regarded as an efficiency of coupling of the freezing medium
to the food varying from 1 for Bi ®
¥,
to 0 for Bi ®
0. Taking an intrinsic freezing time tf’ for
the case of unit driving force, DT
= 1; then for general DT
:
tf = tf' /(hDT)
(6.9)
and
(6.9) can be used very readily to examine the influence of freezing medium
temperature, and of surface heat transfer coefficient through the Biot
number, on the actual freezing time. Thus the sensitivity of freezing
time to process variations can be taken quickly into account.
 EXAMPLE
6.12. Freezing time of a carton of meat: controllable factors EXAMPLE
6.12. Freezing time of a carton of meat: controllable factors
Determine the intrinsic freezing time for the carton of Example 6.11.
By putting the equation for the freezing time in the form of eqn. (6.9)
evaluate the effect of
(a) changes in the temperature of the plates to -20°C, - 25°C,
and -30°C
(b) effect of doubling the thickness of the cardboard and
(c) effect of decreased surface coefficients due to poor contact which
drops the surface heat transfer coefficient to 100 J m-2 s-1
°C-1
Bi = hsa = 52.6 x 0.1
= 3.3
k 1.6
P/R = 4, and so h
= 3.3/7.3 = 0.45
And from Example 6.11, calculated freezing time is 4.2 h with driving
force 32°C
Therefore tf‘ = 4.2 x 0.45 x 32 = 60.5
h,
for (a) tf
= 60.5 /(0.45 x 18) = 7.5 h for -20°C, 60.5/(0.45 x 23) = 5.8
h for -25°C, and 60.5/(0.45 x 28) = 4.8 h for -30°C
for (b) x/k becomes 0.034, therefore 1/hs = 0.034+0.0017
hs = 28 J m-2 s-1 °C-1
Bi becomes (28 x 0.1)/1.6 = 1.75
Therefore h
becomes 0.30
so
tf = 60.5 x 1/0.30 + 1/32
= 6.3 h
for (c) 1/ hs
becomes 0.017 + 0.01 = 0.027,
hs = 37 J m-2 s-1 °C-1
Bi becomes (37 x 0.1)/1.6 = 2.3
Therefore h
becomes 0.37
so
tf = 60.5 x 1/32 x 1/0.37
= 5.1 h
Cold Storage
For cold storage,
the requirement for refrigeration comes from the need to remove the heat:
 coming
into the store from the external surroundings through insulation coming
into the store from the external surroundings through insulation
 from
sources within the store such as motors, lights and people (each worker
contributing something of the order of 0.5 kW) from
sources within the store such as motors, lights and people (each worker
contributing something of the order of 0.5 kW)
 from
the foodstuffs. from
the foodstuffs.
Heat
penetrating the walls can be estimated, knowing the overall heat-transfer
coefficients including the surface terms and the conductances of the insulation,
which may include several different materials. The other heat sources
require to be considered and summed. Detailed calculations can be quite
complicated but for many purposes simple methods give a reasonable estimate.
 Heat-Transfer
Applications > SUMMARY, PROBLEMS Heat-Transfer
Applications > SUMMARY, PROBLEMS
 Back
to the top Back
to the top |
![]() Refrigeration
Cycle
Refrigeration
Cycle![]() Performance
Characteristics
Performance
Characteristics![]() Refrigerants
Refrigerants![]() Mechanical Equipment
Mechanical Equipment![]() The
Refrigeration Evaporator
The
Refrigeration Evaporator![]() Chilling
Chilling![]() Freezing
Freezing![]() Cold Storage
Cold Storage 




