PROBLEMS
1.
If 5 kg of sucrose are dissolved in 20 kg of water estimate the concentration
of the solution in (a) w/w, (b) w/v, (c) mole fraction, (d) molar concentration.
The density of a 20% sucrose solution is 1070 kg m-3, molecular
weight of sucrose 342
(a)
20%, (b) 21.4% (c) 0.018 (d) 0.63 mol m-3
2.
If 1 m3 of air at a pressure of 1 atm is mixed with 0.1 m3
of carbon dioxide at 1.5 atm and the mixture is compressed so that its
total volume is 1 m3, estimate the concentration of the carbon
dioxide in the mixture in (a) w/w, (b) w/v, (c) mole fraction at a temperature
of 25°C. Mean molecular weight of air is 28.8, and of carbon dioxide
44. (a) 18.6%, (b) 27%, (c) 0.13
3.
It is convenient to add salt to butter, produced in a continuous buttermaking
machine, by adding a slurry of salt with water containing 60% of salt
and 40% of water by weight. If the final composition of the butter is
to be 15.8% moisture and 1.4% salt, estimate the original moisture content
of the butter prior to salting.(15.2%)
4.
In a flour mill, wheat is to be adjusted to a moisture content of 15%
on a dry basis. If the whole grain received at the mill is found to contain
11.4% of water initially, how much water must the miller add per 100 kg
of input grain as received, to produce the desired moisture content? (1.8
kg/ 100 kg)
5.
(a) In an analysis, sugar beet is found to contain 75% of water and 17.5%
of sugar. Of the remaining material, 25% is soluble and 75% insoluble,
calculate the sugar content of the expressible juice assumed to contain
water and all soluble solids pro rata.
(b) If the beets are extracted by addition of a weight of water equal
to their own weight and after a suitable period the soluble constituents
are concentrated evenly throughout all the water present, calculate the
percentage of the total sugar left in the drained beet and the percentage
of the total sugar extracted, assuming that the beet cells (insoluble)
after the extraction have the same quantity of water associated with them
as they did in the original beet.
(c) Lay out a mass balance for the extraction process
(a) 18.5% (b) drained beet 43%, extract 57%
6.
A sweet whey, following cheesemaking, has the following composition: 5.5%
lactose, 0.8% protein. 0.5% ash. The equilibrium solubility of lactose
in water is:
 |
Temp. °C |
0
|
15
|
25
|
39
|
49
|
64
|
| |
Lactose solubility
kg/l00 kg water |
11.9
|
16.9
|
21.6
|
31.5
|
42.4
|
65.8
|
Calculate
the percentage yield of lactose when 1000 kg of whey is concentrated in
a vacuum evaporator at 60°C to 60% solids and the concentrate is then
cooled with crystallization of the lactose, down to 20°C over a period
of weeks.
(83.6%)
7.
In an ultrafiltration plant, whey is to be concentrated. Two streams are
to be produced, a protein rich stream and a liquid (mainly water) stream.In
all 140,000 kg per day are to be processed to provide a 12-fold concentration
of 95% of the protein from an original whey concentration of 0.93% protein
and 6% of other soluble solids.
Assuming that all of the soluble solids other than protein remain with
the liquid stream, which also has the 5% of the protein in it, estimate
the daily flows and concentrations of the two product streams.
((a) protein stream: 11,084 kg day-1,11.16% protein,(b) liquid
stream 128,916 kgday-1, 0,05% protein)
8.
It is desired to prepare a sweetened concentrated orange juice. The initial
pressed juice contains 5 % of total solids and it is desired to lift this
to 10% of total solids by evaporation and then to add sugar to give 2%
of added sugar in the concentrated juice. Calculate the quantity of water
which must be removed, and of sugar which must be added with respect to
each 1000 kg of pressed juice.
(water removed 500 kg,sugar added 10.2 kg)
9.
A tomato-juice evaporator takes in juice at the rate of 1200 kg h-1.
If the concentrated juice contains 35% of solids and the hourly rate of
removal of water is 960 kg, calculate the moisture content of the original
juice and the quantity of steam needed per hour for heating if the evaporator
works at a pressure of 10 kPa and the heat available from the steam is
2200 kJ kg-1. Assume no heat losses.
((a) water content of juice, 93%, (b) steam needed 1089 kg h-1))
10. Processing
water is to be heated in a direct fired heater, which burns natural
gas with a calorific value of 20.2 MJ m-3. If 5000 kg h-1
of this water has to be heated from 15°C to 80°C and the heater
is estimated to be 45% efficient, estimate the hourly consumption of
gas.
150 m3.
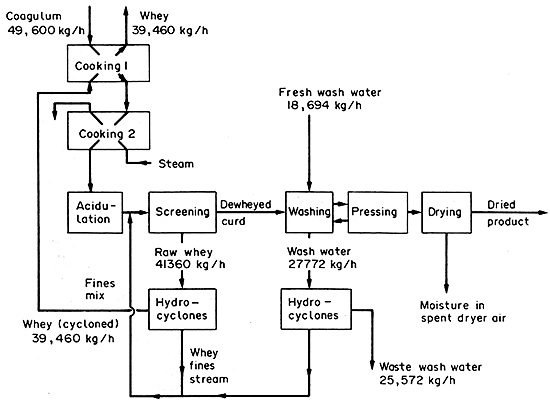
Figure 2.3 Casein process
11. In a casein factory (see Fig. 2.3) the entering coagulum
containing casein and lactose is passed through two cookers and acidified
to precipitate the casein. The casein separates as a curd. The curd
is removed pressed from the whey by screening, and then washed, and
dried. The casein fines are removed from the raw whey from screening
and the wash water by hydrocyclones, and mixed with the heated coagulum
just before screening. The cycloned whey is used for heating in the
first cooker and steam in the second cooker by indirect heating. The
casein and lactose contents of the various streams were determined.
| |
%
Composition
on Wet Weight Basis
|
| |
Casein
|
Lactose
|
Moisture
|
| Coagulum |
2.76
|
3.68
|
|
| Raw
whey from screening |
0.012
|
4.1
|
|
| Whey
(cycloned) |
0.007
|
|
|
| Wash
water |
0.026
|
0.8
|
|
| Waste
wash water |
0.008
|
|
|
| Dried
product |
|
|
11.9
|
From
these data calculate complete mass balances for the process, using a
simple step-by-step approach, starting with the hydrocyclones. Assume
lactose completely soluble in all solutions. and concentrations in fines
and wastes streams from hydrocyclones are the same.
(a)
Set out an overall mass balance for the complete process to the
production of the wet curd. i.e. until. after the washing/pressing operation.
Start the mass balance at the hydrocyclones i.e. mass balances for the
following unit operations: hydrocyclones, cooking, screening,washing;
and then an overall balance.
(b) Set out a casein mass balance over the same unit operations,
and overall. Assume that there was little or no casein removed from
the whey cyclones, and lost from the coagulum.
(c) Set out a lactose balance on the screening and washing.
(d) Set out a mass balance from the wet curd to the dried product. Assume
that the product contains only casein, lactose and water.
(e) What was the composition of the wet curd? (41.8% casein, 0.3% lactose,
57..9% water)
(f) What was the composition of the final product? (87.4% casein,
0.69% lactose, 11.9% water)
(g) Determine the yield of the casein from the coagulum entering, and
the losses in the cycloned whey. and waste water (99.6%,
in waste whey 0.2%, in waste water 0.2%)
 CHAPTER
3: FLUID FLOW THEORY CHAPTER
3: FLUID FLOW THEORY
 Back
to the top Back
to the top |

