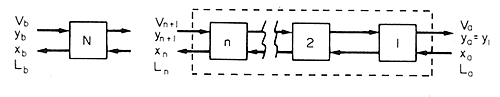
Figure 9.2 Contact equilibrium stages
Taking a mass
balance over the first n stages as shown in Figure 9.2 (right hand
side) we can write, for the total flow, mass entering must equal mass
leaving, so:
Vn+1 + La = Va
+ Ln
and for the component being exchanged:
Vn+1 yn+1 + Laxa = Vaya + Lnxn
where
V is the mass flow rate of the light stream, L is the flow
rate of the heavy stream, y is the concentration of the component
being exchanged in the light stream and x is the concentration
of the component being exchanged in the heavy stream. In the case of the
subscripts, n denotes conditions at equilibrium in the nth stage, n +
1 denotes conditions at equilibrium in the (n + 1)th stage and a
denotes the conditions of the streams entering and leaving stage 1, one
being raw material and one product from that stage
Eliminating Vn+1
between these equations, we have:
Vn+1 = Ln
- La + Va
and so,
yn+1(Ln
- La + Va ) = Vaya + Lnxn
- Laxa
yn+1 = xn
[Ln / (Ln - La+ Va)]
+ [(Vaya
- Laxa)/ (Ln - La
+ Va)] (9.5)
This
is an important equation as it expresses the concentration in one stream
in the (n + 1)th stage in terms of the concentration in the other streams
in the nth stage. In many practical cases in which equal quantities, or
equal molar quantities, of the carrying streams move from one stage to
another, that is where the flow rates are the same in all contact stages,
then for:
lighter phase Ln+1
= Ln = ... La = L
heavier phase Vn+1 =… = Va. = V
A simplified equation
can be written for such cases:
yn+1
= xnL
/ V + ya
- xaL
/ V (9.6)
 Contact-Equilibrium
Processes - THEORY > CALCULATION OF SEPARATION IN CONTACT-EQUILIBRIUM
PROCESSES Contact-Equilibrium
Processes - THEORY > CALCULATION OF SEPARATION IN CONTACT-EQUILIBRIUM
PROCESSES
 Back
to the top Back
to the top |

