The
total pressure of a gaseous mixture, such as air and water vapour, is
made up from the sum of the pressures of its constituents, which are called
the partial pressures. Each partial pressure arises from the molecular
concentration of the constituent and the pressure exerted is that which
corresponds to the number of moles present and the total volume of the
system. The partial pressures are added to obtain the total pressure.
 EXAMPLE
7.5. Partial pressure of water vapour EXAMPLE
7.5. Partial pressure of water vapour
If the total pressure of moist air is 100 kPa (approximately atmospheric)
and the humidity is measured as 0.03 kg kg-1, calculate the
partial pressure of the water vapour.
The molecular weight
of air is 29, and of water 18
So the mole fraction of water = (0.03/18)/(1.00/29 + 0.03/18)
=
0.0017/(0.034 + 0.0017)
=
0.048
Therefore the water
vapour pressure
= 0.048 x 100 kPa
= 4.8 kPa.
The relative humidity (RH) is defined as the ratio of the partial
pressure of the water vapour in the air (p) to the partial pressure
of saturated water vapour at the same temperature (ps).
Therefore:
RH = p/ps
and is often expressed
as a percentage = 100 p/ps
 EXAMPLE
7.6. Relative humidity EXAMPLE
7.6. Relative humidity
If the air in Example 7.5 is at 60°C, calculate the relative humidity.
From steam tables,
the saturation pressure of water vapour at 60°C is 19.9 kPa.
Therefore the relative humidity = p/ps
= 4.8/19.9
= 0.24
or 24%.
If
such air were cooled, then when the percentage relative humidity reached
100% the air would be saturated and this would occur at that temperature
at which p = ps = 4.8 kPa.
Interpolating
from the steam tables, or reading from the water vapour pressure/temperature
graph, this occurs at a temperature of 32°C and this temperature is
called the dew point of the air at this particular moisture content.
If cooled below the dew point, the air can no longer retain this quantity
of water as vapour and so water must condense out as droplets or a fog,
and the water remaining as vapour in the air will be that corresponding
to saturation at the temperature reached.
The
humidity Y can therefore be related to the partial pressure pw
of the water vapour in air by the equation:
Y = 18 pw
/[29(P – pw)]
(7.4)
where
P is the total pressure. In circumstances where pw
is small compared with P, and this is approximately the case in
air/water systems at room temperatures, Y »
18pw/29P.
Corresponding
to the specific heat, cp, of gases, is the humid
heat, cs of moist air. It is used in the same way
as a specific heat, the enthalpy change being the mass of dry air multiplied
by the temperature difference and by the humid heat. The units are J kg-1
°C-1 and the numerical values can be read off a psychrometric
chart. It differs from specific heat at constant pressure in that it is
based only on the mass of the dry air. The specific heat of the water
it contains is effectively incorporated into the humid heat which therefore
is numerically a little larger than the specific heat to allow for this.
Wet-bulb Temperatures
A useful concept in psychrometry is the wet-bulb temperature, as compared
with the ordinary temperature, which is called the dry-bulb temperature.
The wet-bulb temperature is the temperature reached by a water surface,
such as that registered by a thermometer bulb surrounded by a wet wick,
when exposed to air passing over it. The wick and therefore the thermometer
bulb decreases in temperature below the dry-bulb temperature until the
rate of heat transfer from the warmer air to the wick is just equal to
the rate of heat transfer needed to provide for the evaporation of water
from the wick into the air stream.
Equating these two
rates of heat transfer gives
hcA(Ta
- Ts) = lk'gA(Ys–
Ya)
hc(Ta - Ts)
= lk'g(Ys–
Ya)
where
a and s denote actual and saturation temperatures and
humidities; hc is the heat-transfer coefficient and
k'g the mass transfer coefficient from the air to the
wick surface; l is
the latent heat of evaporation of water.
As
the relative humidity of the air decreases, so the difference between
the wet-bulb and dry-bulb temperatures, called the wet-bulb depression,
increases and a line connecting wet-bulb temperature and relative humidity
can be plotted on a suitable chart. When the air is saturated, the wet-bulb
temperature and the dry-bulb temperature are identical.
Therefore
if (Ta– Ts) is plotted against
(Ys– Ya) remembering that the
point (Ts, Ys) must correspond to
a dew-point condition, we then have a wet-bulb straight line on a temperature/humidity
chart sloping down from the point (Ts, Ys)
with a slope of:
- (l
k'g/hc)
A
further important concept is that of the adiabatic saturation condition.
This is the situation reached by a stream of water, in contact with the
humid air, and where the temperature of the air and the humidity follow
down a line called the adiabatic saturation line. Both ultimately reach
a temperature at which the heat lost by the humid air on cooling is equal
to the heat of evaporation of the water leaving the stream of water by
evaporation.
Under this condition
with no heat exchange to the surroundings, the total enthalpy change (kJ
kg-1 dry air)
DH
= cs(Ta– Ts)
+ l(Ys–
Ya) = 0
cs = - hc/k"g
where
cs is the humid heat of the air.
Now it just so happens, for the water/air system at normal working temperatures
and pressures that for practical purposes the numerical magnitude of the
ratio:
cs
= - hc/k"g (known as
the Lewis number)
» 1
(7.5)
This
has a useful practical consequence. The wet bulb line and the adiabatic
saturation line coincide when the Lewis number = 1.
It
is now time to examine the chart we have spoken about. It is called a
psychrometric chart.
Psychrometric Charts
In
the preceding discussion, we have been considering a chart of humidity
against temperature, and such a chart is given in skeleton form on Fig.
7.3 and more fully in Appendix 9, (a)
Normal Temperatures and (b) High Temperatures.
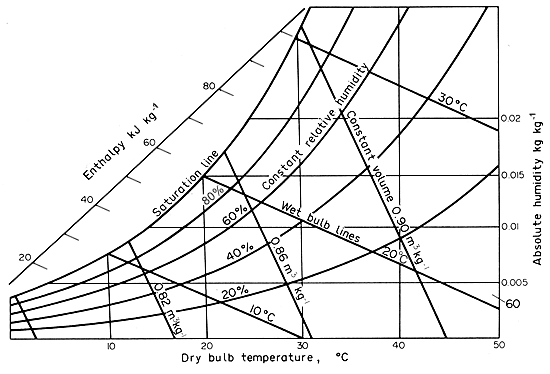
Figure 7.3 Psychrometric chart
The two main axes are temperature (dry bulb) and humidity (humidity ratio)
. The saturation curve (Ts , Ys).
is plotted on this dividing the whole area into an unsaturated and a two-phase
region. Taking a point on the saturation curve (Ts,
Ys) a line can be drawn from this with a slope:
- (lk'g/hc)
= (l/cs)
running
down into the unsaturated region of the chart (that “below”
the saturation line). This is the wet bulb or adiabatic cooling line and
a net of such lines is shown. Any constant temperature line running between
the saturation curve and the zero humidity axis can be divided evenly
into fractional humidities which will correspond to fractional relative
humidities [for example, a 0.50 ratio of humidities will correspond to
a 50% RH because of eqn. (7.4) if P » pw].
This
discussion is somewhat over-simplified and close inspection of the chart
shows that the axes are not exactly rectangular and that the lines of
constant dry-bulb temperature are not exactly parallel. The reasons are
beyond the scope of the present discussion but can be found in appropriate
texts such as Keey (1978). The chart also
contains other information whose use will emerge as familiarity grows.
This
chart can be used as the basis of many calculations. It can be used to
calculate relative humidities and other properties.
 EXAMPLE
7.7. Relative humidity, enthalpy and specific volume of air EXAMPLE
7.7. Relative humidity, enthalpy and specific volume of air
If the wet-bulb temperature in a particular room is measured and found
to be 20°C in air whose dry-bulb temperature is 25°C (that is
the wet-bulb depression is 5°C) estimate the relative humidity, the
enthalpy and the specific volume of the air in the room.
On
the humidity chart (Appendix 9a) follow down the wet-bulb line for a temperature
of 20°C until it meets the dry-bulb temperature line for 25°C.
Examining the location of this point of intersection with reference to
the lines of constant relative humidity, it lies between 60% and 70% RH
and about 4/10 of the way between them but nearer to the 60% line. Therefore
the RH is estimated to be 64%. Similar examination of the enthalpy lines
gives an estimated enthalpy of 57 kJ kg-1, and from the volume
lines a specific volume of 0.862 m3 kg-1.
Once
the properties of the air have been determined other calculations can
easily be made.
 EXAMPLE
7.8. Relative humidity of heated air EXAMPLE
7.8. Relative humidity of heated air
If the air in Example 7.7 is then to be heated to a dry-bulb temperature
of 40°C, calculate the rate of heat supply needed for a flow of 1000
m3 h-1 of this hot air for a dryer, and the relative
humidity of the heated air.
On
heating, the air condition moves, at constant absolute humidity as no
water vapour is added or subtracted, to the condition at the higher (dry
bulb) temperature of 40°C. At this condition, reading from the chart
at 40°C and humidity 0.0125 kg kg-1, the enthalpy is 73
kJ kg-1, specific volume is 0.906 m3 kg-1
and RH 27%.
Mass of 1000 m3
is 1000/0.906 = 1104kg,
DH
= (73 - 57) = 16 kJ kg-1.
So rate of heating required
= 1104 x 16 kJ h -1
= (1104 x 16)/3600 kJ s -1
= 5 kW
If the air is used for drying, with the heat for evaporation being supplied
by the hot air passing over a wet solid surface, the system behaves like
the adiabatic saturation system. It is adiabatic because no heat is obtained
from any source external to the air and the wet solid, and the latent
heat of evaporation must be obtained by cooling the hot air. Looked at
from the viewpoint of the solid, this is a drying process; from the viewpoint
of the air it is humidification.
 EXAMPLE
7.9. Water removed in air drying EXAMPLE
7.9. Water removed in air drying
Air at 60°C and 8% RH is blown through a continuous dryer from which
it emerges at a temperature of 35°C. Estimate the quantity of water
removed per kg of air passing, and the volume of drying air required to
remove 20 kg water per hour.
Using
the psychrometric chart (high-temperature version,
Appendix 9(b) to take in the conditions), the inlet air condition shows
the humidity of the drying air to be 0.01 kg kg-1 and its specific
volume to be 0.96 m3 kg-1. Through the dryer, the
condition of the air follows a constant wet-bulb line of about 27°C
, so at 35°C its condition is a humidity of 0.0207kg kg-1.
Water removed
= (0.0207 - 0.010)
= 0.0107 kg kg-1 of air.
So each kg, i.e.
0.96 m3, of air passing will remove 0.0107kg water,
Volume of air
to remove 20 kg h-1
= (20/0.0107) x 0.96
= 1794 m3
h-1
If air is cooled, then initially its condition moves along a line of constant
humidity, horizontally on a psychrometric chart, until it reaches the
saturation curve at its dew point. Further cooling then proceeds down
the saturation line to the final temperature, with water condensing to
adjust the humidity as the saturation humidity cannot be exceeded.
 EXAMPLE
7.10. Relative humidity of air leaving a dryer EXAMPLE
7.10. Relative humidity of air leaving a dryer
The air emerging from a dryer, with an exit temperature of 45°C, passes
over a surface which is gradually cooled. It is found that the first traces
of moisture appear on this surface when it is at 40°C. Estimate the
relative humidity of the air leaving the dryer.
On
the psychrometric chart, the saturation temperature is 40°C and proceeding
at constant humidity from this, the 45°C line is intersected at a
point indicating:
relative humidity = 76%
In
dryers, it is sometimes useful to reheat the air so as to reduce its relative
humidity and thus to give it an additional capacity to evaporate more
water from the material being dried. This process can easily be followed
on a psychrometric chart.
 EXAMPLE
7.11. Reheating of air in a dryer EXAMPLE
7.11. Reheating of air in a dryer
A flow of 1800 m3 h-1 of air initially at a temperature
of 18°C and 50% RH is to be used in an air dryer. It is heated to
140°C and passed over a set of trays in a shelf dryer, which it leaves
at 60 % RH. It is then reheated to 140°C and passed over another set
of trays which it leaves at 60 % RH again. Estimate the energy necessary
to heat the air and the quantity of water removed per hour.
From
the psychrometric chart [normal temperatures,
Appendix 9(a)] the humidity of the initial air is 0.0062 kg kg-1,
specific volume is 0.834 m3 kg-1, and enthalpy 35
kJ kg-1. Proceeding at constant humidity to a temperature of
140°C, the enthalpy is found (high temperature chart) to be 160 kJ
kg-1. Proceeding along a wet-bulb line to an RH of 60% gives
the corresponding temperature as 48°C and humidity as 0.045 kg kg-1.
Reheating to 140°C
keeps humidity constant and enthalpy goes to 268 kJ kg-1.
Thence along a wet-bulb
line to 60 % RH gives humidity of 0.082 kg kg-1.
Total energy supplied
= DH
in heating and reheating
= 268 - 35
= 233 kJ kg-1
Total water removed
= DY
= 0.082 - 0.0062
= 0.0758 kg kg-1
1800 m3
of air per hour = 1800/0.834
= 2158 kg h-1
= 0.6 kg s-1
Energy taken in
by air = 233 x 0.6 kJ s-1
= 140 kW
Water removed
in dryer = 0.6 x 0.0758
= 0.045 kg s-1
= 163kgh -1
Exit
temperature of air (from chart) = 60°C.
Consideration of psychrometric charts, and what has been said about them,
will show that they can be used for calculations focused on the air, for
the purposes of air conditioning as well as for drying.
 EXAMPLE
7.12. Air conditioning EXAMPLE
7.12. Air conditioning
In a tropical country, it is desired to provide processing air conditions
of 15°C and 80% RH. The ambient air is at 31.5°C and 90% RH. If
the chosen method is to cool the air to condense out enough water to reduce
the water content of the air sufficiently, then to reheat if necessary,
determine the temperature to which the air should be cooled, the quantity
of water removed and the amount of reheating necessary. The processing
room has a volume of 1650 m3 and it is estimated to require
six air changes per hour.
Using the psychrometric
chart (normal temperatures):
Initial humidity is 0.0266 kg kg-1.
Final humidity is 0.0085 kg kg-1.
Saturation temperature for this humidity is 13°C.
Therefore the air should be cooled to 13°C
At the saturation temperature of 13°C, the enthalpy is 33.5 kJ kg-1
At the final conditions, 15°C and 80 % RH, the enthalpy is 37 kJ
kg-1
and the specific volume of air is 0.827 m3 kg-1.
Assuming that the
air changes are calculated at the conditions in the working space.
Mass of air to be conditioned = (1650
x 6)/0.827
= 11,970 kg h-1
Water removed per kg of dry air DY
= 0.0266 - 0.0085
= 0.018 kg kg-1
Mass of water removed per hour
= 11,970 x 0.018
= 215 kg h-1
Reheat required DH
=
(37 - 33.5)
= 3.5 kJ kg-1
Total reheat power required
= 11,970 x 3.5
= 41,895 kJ h-1
= 11.6 kJ s-1
= 11.6 kW.
Measurement of Humidity
Methods depend largely upon the concepts that have been presented in the
preceding sections, but because they are often needed it seems useful
to set them out specifically. Instruments for the measurement of humidity
are called hygrometers.
 Wet-
and dry-bulb thermometers. The dry-bulb temperature is the normal air
temperature and the only caution that is needed is that if the thermometer
bulb, or element, is exposed to a surface at a substantially higher
or lower temperature the possibility of radiation errors should be considered.
A simple method to greatly reduce any such error is to interpose a radiation
shield, e.g. a metal tube, which stands off from the thermometer bulb
1 cm or so and prevents direct exposure to the radiation source or sink.
For the wet bulb thermometer, covering the bulb with a piece of wicking,
such as a hollow cotton shoelace of the correct size, and dipping the
other end of the wick into water so as to moisten the wet bulb by capillary
water flow, is adequate. The necessary aspiration of air past this bulb
can be effected by a small fan or by swinging bulb, wick, water bottle
and all through the air, as in a sling psychrometer. The maximum difference
between the two bulbs gives the wet-bulb depression and a psychrometric
chart or appropriate tables will then give the relative humidity. Wet-
and dry-bulb thermometers. The dry-bulb temperature is the normal air
temperature and the only caution that is needed is that if the thermometer
bulb, or element, is exposed to a surface at a substantially higher
or lower temperature the possibility of radiation errors should be considered.
A simple method to greatly reduce any such error is to interpose a radiation
shield, e.g. a metal tube, which stands off from the thermometer bulb
1 cm or so and prevents direct exposure to the radiation source or sink.
For the wet bulb thermometer, covering the bulb with a piece of wicking,
such as a hollow cotton shoelace of the correct size, and dipping the
other end of the wick into water so as to moisten the wet bulb by capillary
water flow, is adequate. The necessary aspiration of air past this bulb
can be effected by a small fan or by swinging bulb, wick, water bottle
and all through the air, as in a sling psychrometer. The maximum difference
between the two bulbs gives the wet-bulb depression and a psychrometric
chart or appropriate tables will then give the relative humidity.
 Dew-point
meters. These measure the saturation or dew-point temperature by cooling
a sample of air until condensation occurs. The psychrometric chart or
a scale on the instrument is then used to give the humidity. For example,
a sample of air at 20°C is found to produce the first signs of condensation
on a mirror when the mirror is cooled to 14°C. The chart shows by
moving horizontally across, from the saturation temperature of 14°C
to the constant temperature line at 20°C, that the air must have
a relative humidity of 69%. Dew-point
meters. These measure the saturation or dew-point temperature by cooling
a sample of air until condensation occurs. The psychrometric chart or
a scale on the instrument is then used to give the humidity. For example,
a sample of air at 20°C is found to produce the first signs of condensation
on a mirror when the mirror is cooled to 14°C. The chart shows by
moving horizontally across, from the saturation temperature of 14°C
to the constant temperature line at 20°C, that the air must have
a relative humidity of 69%.
 The
hair hygrometer. Hairs expand and contract in length according to the
relative humidity. Instruments are made which give accurately the length
of the hair and so they can be calibrated in humidities. The
hair hygrometer. Hairs expand and contract in length according to the
relative humidity. Instruments are made which give accurately the length
of the hair and so they can be calibrated in humidities.
 Electrical
resistance hygrometers. Some materials vary in their surface electrical
resistance according to the relative humidity of the surrounding air.
Examples are aluminium oxide, phenol formaldehyde polymers, and styrene
polymers. Calibration allows resistance measurements to be interpreted
as humidity. Electrical
resistance hygrometers. Some materials vary in their surface electrical
resistance according to the relative humidity of the surrounding air.
Examples are aluminium oxide, phenol formaldehyde polymers, and styrene
polymers. Calibration allows resistance measurements to be interpreted
as humidity.
 Lithium
chloride hygrometers. In these a solution of lithium chloride is brought
to a temperature such that its partial pressure equals the partial pressure
of water vapour in the air. The known vapour pressure-temperature relationships
for lithium chloride can then be used to determine the humidity of the
air. Lithium
chloride hygrometers. In these a solution of lithium chloride is brought
to a temperature such that its partial pressure equals the partial pressure
of water vapour in the air. The known vapour pressure-temperature relationships
for lithium chloride can then be used to determine the humidity of the
air.
 Drying
> EQUILIBRIUM MOISTURE CONTENT Drying
> EQUILIBRIUM MOISTURE CONTENT
 Back
to the top Back
to the top |
![]() Wet-bulb
Temperatures
Wet-bulb
Temperatures![]() Psychrometric
Charts
Psychrometric
Charts ![]() Measurement
of Humidity
Measurement
of Humidity
