FLUID STATICS
A very important property of a fluid at rest is the pressure exerted by
that fluid on its surroundings, that is the fluid pressure
Pressure
is defined as force exerted on an area. Under the influence of gravity,
a mass of any material exerts a force on whatever supports it. The magnitude
of this force is equal to the mass of the material multiplied by the acceleration
due to gravity. The mass of a fluid can be calculated by multiplying its
volume by its density, which is defined as its mass per unit volume. Thus
the equation can be written:
F = mg = Vrg
where F
is the force exerted, m is the mass, g the acceleration
due to gravity, V the volume and r
(the Greek letter rho) the density. The units of force are Newtons,
or kg m s-2. and of pressure Pascals, one Pascal being one
Newton m-2, and so one Pascal is also one kg m-1
s-2.
For a mass to remain
in equilibrium, the force it exerts due to gravity must be resisted
by some supporting medium. For a weight resting on a table, the table
provides the supporting reaction; for a multi-storey building, the upper
floors must be supported by the lower ones so that as you descend the
building the burden on the floors increases until the foundations support
the whole building. In a fluid, the same situation applies.
Lower
levels of the fluid must provide the support for the fluid that lies above
them. The fluid at any point must support the fluid above. Also, since
fluids at rest are not able to sustain shearing forces, which are forces
tending to move adjacent layers in the fluid relative to one another,
it can be shown that the forces at any point in a fluid at rest are equal
in all directions. The force per unit area in a fluid is called the fluid
pressure. It is exerted equally in all directions.
Consider a horizontal
plane in a fluid at a depth Z below the surface, as illustrated
in Fig. 3.1.
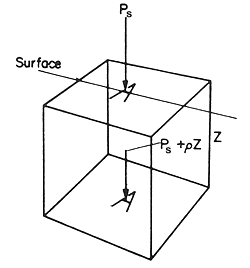
Figure 3.1 Pressure in a fluid
If
the density of the fluid is r,
then the volume of fluid lying above an area A on the plane is
ZA and the weight of this volume of fluid, which creates a force
exerted by it on the area A which supports it, is ZrAg.
But the total force on the area A must also include any additional
force on the surface of the liquid. If the force on the surface is Ps
per unit area,
F = APs
+ ZrAg
(3.1)
where
F is the total force exerted on the area A and Ps
is the pressure above the surface of the fluid (e.g. it might be atmospheric
pressure). Further, since total pressure P is the total force per
unit area,
P = F/A = Ps
+ Zrg
(3.2)
In
general, we are interested in pressures above or below atmospheric. If
referred to zero pressure as datum, the pressure of the atmosphere must
be taken into account. Otherwise the atmospheric pressure represents a
datum or reference level from which pressures are measured. In these circumstances
we can write:
P = Zrg
(3.3)
This
may be considered as the fundamental equation of fluid pressure. It states
that the product of the density of the fluid, acceleration due to gravity
and the depth gives the pressure at any depth in a fluid.
 EXAMPLE
3.1. Total pressure in a tank of peanut oil EXAMPLE
3.1. Total pressure in a tank of peanut oil
Calculate the greatest pressure in a spherical tank, of 2 m diameter,
filled with peanut oil of specific gravity 0.92, if the pressure measured
at the highest point in the tank is 70 kPa.
Density of water
= 1000 kg m-3
Density of oil = 0.92 x 1000
kg m-3 = 920 kg m-3
Z =greatest depth = 2 m
and g
= 9.81 m s-2
Now P = Zrg
=
2 x 920 x 9.81 kg m-1 s-2
=
18,050 Pa = 18.1 kPa.
To this must be added
the pressure at the surface of 70 kPa.
Total pressure =
70 + 18.1 = 88.1 kPa.
Note
in Example 3.1, the pressure depends upon the pressure at the top
of the tank added to the pressure due to the depth of the liquid; the
fact that the tank is spherical (or any other shape) makes no difference
to the pressure at the bottom of the tank.
In
the previous paragraph, we established that the pressure at a point in
a liquid of a given density is solely dependent on the density of the
liquid and on the height of the liquid above the point, plus any pressure
which may exist at the surface of the liquid. When the depths of the fluid
are substantial, fluid pressures can be considerable.
For example, the pressure on a
plate 1 m2 lying at a depth of 30 m will be the weight of 1
m3 of water multiplied by the depth of 30 m and this will amount
to 30 x 1000 x 9.81 = 294.3 kPa. As 1 tonne exerts a force on 1 m2
of 1000 x 9.81 = 9810 Pa = 9.81 kPa the pressure on the plate is equal
to that of a weight of 294.3 / 9.81 = 30 tonnes of water.
Pressures
are sometimes quoted as absolute pressures and this means the total
pressure including atmospheric pressure. More usually, pressures are given
as gauge pressures, which implies the pressure above atmospheric
pressure as datum. For example, if the absolute pressure is given as 350
kPa, the gauge pressure is (350 - 100) = 250 kPa assuming that the atmospheric
pressure is 100 kPa. These pressure conversions are illustrated in Fig.
3.2.
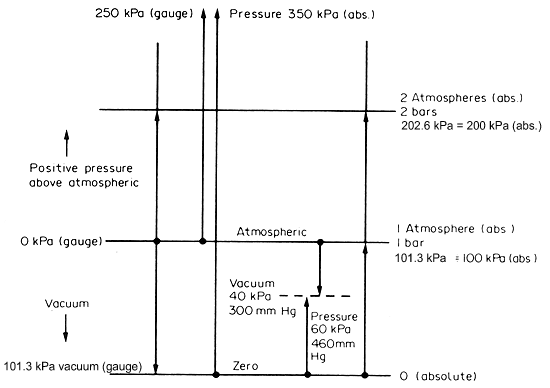
Figure 3.2 Pressure conversions
Standard atmospheric pressure is actually 101.3 kPa
but for our practical purposes 100 kPa is sufficiently close and most
convenient to use. Any necessary adjustment can easily be made.
Another
commonly used method of expressing pressures is in terms of "head"
of a particular fluid. From eqn. (3.3) it can be seen that there is a
definite relationship between pressure and depth in a fluid of given density.
Thus pressures can be expressed in terms of depths, or heads as they are
usually called, of a given fluid.
The two fluids most commonly used, when expressing pressures in this way,
are water and mercury. The main reason for this method of expressing pressures
is that the pressures themselves are often measured by observing the height
of the column of liquid that the pressure can support. It is straightforward
to convert pressures expressed in terms of liquid heads to equivalent
values in kPa by the use of eqn. (3.3.).
 EXAMPLE
3.2. Head of Water EXAMPLE
3.2. Head of Water
Calculate the head of water equivalent to standard atmospheric pressure
of 100 kPa.
Density of water
= 1000 kg m-3,
g = 9.81 m s-2
and pressure = 100 kPa
=
100 x 103 Pa = 100 x 103 kg
m-1s-2.
but from eqn. (3.3):
Z
= P/rg
=
(100 x 103)/ (1000 x 9.81)
=
10.2 m
 EXAMPLE
3.3. Head of mercury EXAMPLE
3.3. Head of mercury
Calculate the head of mercury equivalent to a pressure of two atmospheres.
Density of mercury
= 13,600 kg m-3
Z
= (2 x 100 x 103)/
(13,600 x 9.81)
=
1.5m
 Fluid-flow
theory > FLUID DYNAMICS Fluid-flow
theory > FLUID DYNAMICS
 Back
to the top Back
to the top |


