As the concentrations
rise, the viscosity of the liquor also rises. The increase in the viscosity
of the liquor affects the heat transfer and it often imposes a limit on
the extent of evaporation that is practicable.
There is no straightforward
method of predicting the extent of the boiling-point elevation in the
concentrated solutions that are met in some evaporators in practical situations.
Many solutions have their boiling points at some concentrations tabulated
in the literature, and these can be extended by the use of a relationship
known as Duhring's rule. Duhring's rule states
that the ratio of the temperatures at which two solutions (one of which
can be pure water) exert the same vapour pressure is constant.
Thus, if we take the vapour pressure/temperature relation of a reference
liquid, usually water and if we know two points on the vapour pressure-temperature
curve of the solution that is being evaporated, the boiling points of
the solution to be evaporated at various pressures can be read off from
the diagram called a Duhring plot. The Duhring plot will
give the boiling point of solutions of various concentrations by interpolation,
and at various pressures by proceeding along a line of constant composition.
A Duhring plot of the boiling points of sodium chloride solutions is given
in Fig. 8.3.
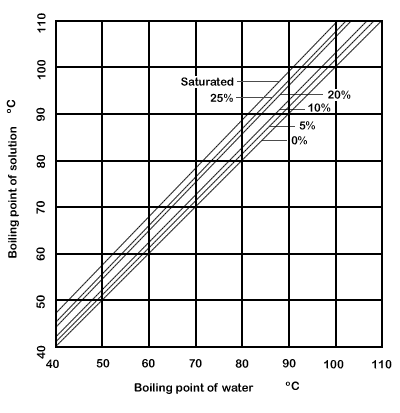
Figure 8.3 Duhring plot for boiling point of sodium chloride solutions
 EXAMPLE
8.5. Duhring Plot for sodium chloride solutions EXAMPLE
8.5. Duhring Plot for sodium chloride solutions
It is found that a saturated solution of sodium chloride in water boils
under atmospheric pressure at 109°C. Under an absolute pressure of
25.4 kPa, water boils at 65.6°C and saturated sodium chloride at 73.3°C.
From these, draw a Duhring plot for saturated salt solution. Knowing the
vapour pressure/temperature relationship for water from Fig.
7.2, find the boiling temperature of saturated salt solution under
a total pressure of 33.3kPa. The Duhring plot
for salt solution has been given in Fig. 8.3, and since the line is straight,
it may be seen that knowledge of two points on it, and the corresponding
boiling points for the reference substance, water, would enable the line
to be drawn.
From the line, and
using Fig. 7.2 again, it is found that:
the boiling point of water under a pressure of 33.3 kPa is 71.7°C,
we can read off the corresponding boiling temperature for saturated salt
solution as 79.4°C.
By finding the boiling
points of salt solutions of various concentrations under two pressures,
the Duhring lines can then, also, be filled in for solutions of these
concentrations. Such lines are also on Fig.8.3. Intermediate concentrations
can be estimated by interpolation and so the complete range of boiling
points at any desired concentration, and under any given pressure, can
be determined.
Latent
heats of vaporization also increase as pressures are reduced, as shown
for water in Table 7.1. Methods for determining these changes can be found
in
the literature, for example in Perry (1997).
 Evaporation
> EVAPORATION OF HEAT-SENSITIVE MATERIALS Evaporation
> EVAPORATION OF HEAT-SENSITIVE MATERIALS
 Back
to the top Back
to the top |

